Abstract
Objective
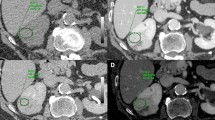
This study aimed to assess material-specific iodine and fat images for diagnosis of clear cell renal cell carcinoma (cc-RCC) compared to papillary RCC (p-RCC) and other renal masses.
Materials and methods
With IRB approval, we identified histologically confirmed solid renal masses that underwent rapid-kVp-switch DECT between 2016 and 2018: 25 cc-RCC (7 low grade versus 18 high grade), 11 p-RCC, and 6 other tumors (2 clear cell papillary RCC, 2 chromophobe RCC, 1 oncocytoma, 1 renal angiomyomatous tumor). A blinded radiologist measured iodine and fat concentration on material-specific iodine–water and fat–water basis pair images. Comparisons were performed between groups using univariate analysis and diagnostic accuracy calculated by ROC.
Results
Iodine concentration was higher in cc-RCC (6.14 ± 1.79 mg/mL) compared to p-RCC (1.40 ± 0.54 mg/mL, p < 0.001), but not compared to other tumors (5.0 ± 2.2 mg/mL, p = 0.370). Intratumoral fat was seen in 36.0% (9/25) cc-RCC (309.6 ± 234.3 mg/mL [71.1–762.3 ng/mL]), 9.1% (1/11) papillary RCC (97.11 mg/mL), and no other tumors (p = 0.036). Iodine concentration ≥ 3.99 mg/mL achieved AUC and sensitivity/specificity of 0.88 (CI 0.76–1.00) and 92.31%/82.40% to diagnose cc-RCC. To diagnose p-RCC, iodine concentration ≤ 2.5 mg/mL achieved AUC and sensitivity/specificity of 0.99 (0.98–1.00) and 100%/100%. The presence of intratumoral fat had AUC 0.64 (CI 0.53–0.75) and sensitivity/specificity of 34.6%/93.8% to diagnose cc-RCC. A logistic regression model combining iodine concentration and presence of fat increased AUC to 0.91 (CI 0.81–1.0) with sensitivity/specificity of 80.8%/93.8% to diagnose cc-RCC.
Conclusion
Iodine concentration values are highly accurate to differentiate clear cell RCC from papillary RCC; however, they overlap with other tumors. Fat-specific images may improve differentiation of clear cell RCC from other avidly enhancing tumors.
Key Points
• Clear cell renal cell carcinoma (RCC) has significantly higher iodine concentration than papillary RCC, but there is an overlap in values comparing clear cell RCC to other renal tumors.
• Iodine concentration ≤ 2.5 mg/mL is highly accurate to differentiate papillary RCC from clear cell RCC and other renal tumors.
• The presence of microscopic fat on material-specific fat images was specific for clear cell RCC, helping to differentiate clear cell RCC from other avidly enhancing renal tumors.






Similar content being viewed by others
Abbreviations
- AS:
-
Active surveillance
- ASiR:
-
Adaptive statistical iterative reconstruction
- AUC:
-
Area under curve
- cc-RCC:
-
Clear cell renal cell carcinoma
- CM phase:
-
Corticomedullary phase
- CS-MRI:
-
Chemical shift MRI
- CT:
-
Computed tomography
- DECT:
-
Dual-energy computed tomography
- GU:
-
Genitourinary
- HU:
-
Hounsfield unit
- ISUP:
-
International Society of Urogenital Pathology
- kVp:
-
Kilovoltage peak
- MRI:
-
Magnetic resonance imaging
- NECT:
-
Nonenhanced contrast tomography
- PACS:
-
Picture Archiving and Communication System
- PPV:
-
Positive predictive value
- RAT:
-
Renal angiomyomatous tumor
- RCC:
-
Renal cell carcinoma
- ROC:
-
Receiver operator characteristic
- ROI:
-
Region of interest
References
Ljungberg B, Bensalah K, Canfield S et al (2015) EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67:913–924
Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MAS (2004) The natural history of incidentally detected small renal masses. Cancer 100:738–745
Herts BR, Silverman SG, Hindman NM et al (2018) Management of the incidental renal mass on CT: a white paper of the ACR Incidental Findings Committee. J Am Coll Radiol 15:264–273
Woo S, Cho JY (2015) Imaging findings of common benign renal tumors in the era of small renal masses: differential diagnosis from small renal cell carcinoma: current status and future perspectives. Korean J Radiol 16:99–113
Ishigami K, Jones AR, Dahmoush L, Leite LV, Pakalniskis MG, Barloon TJ (2015) Imaging spectrum of renal oncocytomas: a pictorial review with pathologic correlation. Insights Imaging 6:53–64
Schieda N, McInnes MD, Cao L (2014) Diagnostic accuracy of segmental enhancement inversion for diagnosis of renal oncocytoma at biphasic contrast enhanced CT: systematic review. Eur Radiol 24:1421–1429
Remzi M, Ozsoy M, Klingler HC et al (2006) Are small renal tumors harmless? Analysis of histopathological features according to tumors 4 cm or less in diameter. J Urol 176:896–899
Sasaguri K, Takahashi N, Gomez-Cardona D et al (2015) Small (< 4 cm) renal mass: differentiation of oncocytoma from renal cell carcinoma on biphasic contrast-enhanced CT. AJR Am J Roentgenol 205:999–1007
Bahouth Z, Halachmi S, Meyer G, Avitan O, Moskovitz B, Nativ O (2015) The natural history and predictors for intervention in patients with small renal mass undergoing active surveillance. Adv Urol 2015
Gordetsky J, Eich M-L, Garapati M, del Carmen Rodriguez Pena M, Rais-Bahrami S (2019) Active surveillance of small renal masses. Urology 123:157–166
Abdel-Rahman O (2018) Impact of histological subtype on outcomes of renal cell carcinoma patients. J Drug Assess 7:14–20
Keegan KA, Schupp CW, Chamie K, Hellenthal NJ, Evans CP, Koppie TM (2012) Histopathology of surgically treated renal cell carcinoma: survival differences by subtype and stage. J Urol 188:391–397
Wang R, Wolf JS Jr, Wood DP Jr, Higgins EJ, Hafez KS (2009) Accuracy of percutaneous core biopsy in management of small renal masses. Urology 73:586–590
Lim CS, Schieda N, Silverman SG (2019) Update on indications for percutaneous renal mass biopsy in the era of advanced CT and MRI. AJR Am J Roentgenol 212:1187–1196
Gellert LL, Mehra R, Chen YB et al (2014) The diagnostic accuracy of percutaneous renal needle core biopsy and its potential impact on the clinical management of renal cortical neoplasms. Arch Pathol Lab Med 138:1673–1679
He Q, Wang H, Kenyon J et al (2015) Accuracy of percutaneous core biopsy in the diagnosis of small renal masses (≤ 4.0 cm): a meta-analysis. Int Braz J Urol 41:15–25
Marconi L, Dabestani S, Lam TB et al (2016) Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 69:660–673
Menogue SR, O'Brien BA, Brown AL, Cohen RJ (2013) Percutaneous core biopsy of small renal mass lesions: a diagnostic tool to better stratify patients for surgical intervention. BJU Int 111:E146–E151
Giménez-Bachs JM, Salinas-Sánchez AS (2019) Improving the diagnosis of renal masses: can we approach the histological diagnosis to the image? Ann Transl Med 7:56–56
Kay FU, Pedrosa I (2017) Imaging of solid renal masses. Radiol Clin North Am 55:243–258
Lopes Vendrami C, Parada Villavicencio C, DeJulio TJ et al (2017) Differentiation of solid renal tumors with multiparametric MR imaging. Radiographics 37:2026–2042
Low G, Huang G, Fu W, Moloo Z, Girgis S (2016) Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol 8:484–500
Kim JK, Kim TK, Ahn HJ, Kim CS, Kim K-R, Cho K-S (2002) Differentiation of subtypes of renal cell carcinoma on helical CT scans. AJR Am J Roentgenol 178:1499–1506
Prasad SR, Humphrey PA, Catena JR et al (2006) Common and uncommon histologic subtypes of renal cell carcinoma: imaging spectrum with pathologic correlation. Radiographics 26:1795–1806
Young JR, Margolis D, Sauk S, Pantuck AJ, Sayre J, Raman SS (2013) Clear cell renal cell carcinoma: discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology 267:444–453
Feng Z, Shen Q, Li Y, Hu Z (2019) CT texture analysis: a potential tool for predicting the Fuhrman grade of clear-cell renal carcinoma. Cancer Imaging 19:6
Zhang GM, Shi B, Xue HD, Ganeshan B, Sun H, Jin ZY (2019) Can quantitative CT texture analysis be used to differentiate subtypes of renal cell carcinoma? Clin Radiol 74:287–294
Krishna S, Sadoughi N, McInnes MDF, Chatelain R, MacDonald DB, Schieda N (2018) Attenuation and degree of enhancement with conventional 120-kVp polychromatic CT and 70-keV monochromatic rapid kilovoltage-switching dual-energy CT in cystic and solid renal masses. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.17.19226:1-8
Marin D, Davis D, Roy Choudhury K et al (2017) Characterization of small focal renal lesions: diagnostic accuracy with single-phase contrast-enhanced dual-energy CT with material attenuation analysis compared with conventional attenuation measurements. Radiology 284:737–747
Mileto A, Allen BC, Pietryga JA et al (2017) Characterization of incidental renal mass with dual-energy CT: diagnostic accuracy of effective atomic number maps for discriminating nonenhancing cysts from enhancing masses. AJR Am J Roentgenol 209:W221–W230
Mileto A, Nelson RC, Paulson EK, Marin D (2015) Dual-energy MDCT for imaging the renal mass. AJR Am J Roentgenol 204:W640–W647
Chandarana H, Megibow AJ, Cohen BA et al (2011) Iodine quantification with dual-energy CT: phantom study and preliminary experience with renal masses. AJR Am J Roentgenol 196:W693–W700
McCollough CH, Leng S, Yu L, Fletcher JG (2015) Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology 276:637–653
Dai C, Cao Y, Jia Y et al (2018) Differentiation of renal cell carcinoma subtypes with different iodine quantification methods using single-phase contrast-enhanced dual-energy CT: areal vs. volumetric analyses. Abdom Radiol (NY) 43:672–678
Mileto A, Marin D, Alfaro-Cordoba M et al (2014) Iodine quantification to distinguish clear cell from papillary renal cell carcinoma at dual-energy multidetector CT: a multireader diagnostic performance study. Radiology 273:813–820
Zarzour JG, Milner D, Valentin R et al (2017) Quantitative iodine content threshold for discrimination of renal cell carcinomas using rapid kV-switching dual-energy CT. Abdom Radiol (NY) 42:727–734
Kay FU, Canvasser NE, Xi Y et al (2018) Diagnostic performance and interreader agreement of a standardized MR imaging approach in the prediction of small renal mass histology. Radiology 287:543–553
Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM (2016) The 2016 WHO classification of tumours of the urinary system and male genital organs—part A: renal, penile, and testicular tumours. Eur Urol 70:93–105
Sadoughi N, Krishna S, Macdonald DB et al (2019) Diagnostic accuracy of attenuation difference and iodine concentration thresholds at rapid-kilovoltage-switching dual-energy CT for detection of enhancement in renal masses. AJR Am J Roentgenol. https://doi.org/10.2214/ajr.18.20990:1-7
Pooler BD, Pickhardt PJ, O'Connor SD, Bruce RJ, Patel SR, Nakada SY (2012) Renal cell carcinoma: attenuation values on unenhanced CT. AJR Am J Roentgenol 198:1115–1120
Matsumoto K, Jinzaki M, Tanami Y, Ueno A, Yamada M, Kuribayashi S (2011) Virtual monochromatic spectral imaging with fast kilovoltage switching: improved image quality as compared with that obtained with conventional 120-kVp CT. Radiology 259:257–262
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Kaza RK, Ananthakrishnan L, Kambadakone A, Platt JF (2017) Update of dual-energy CT applications in the genitourinary tract. AJR Am J Roentgenol 208:1185–1192
Salameh J-P, McInnes MDF, McGrath TA, Salameh G, Schieda N (2019) Diagnostic accuracy of dual-energy CT for evaluation of renal masses: systematic review and meta-analysis. AJR Am J Roentgenol 212:W100–W105
Mileto A, Marin D, Ramirez-Giraldo JC et al (2014) Accuracy of contrast-enhanced dual-energy MDCT for the assessment of iodine uptake in renal lesions. AJR Am J Roentgenol 202:W466–W474
Connolly MJ, McInnes MDF, El-Khodary M, McGrath TA, Schieda N (2017) Diagnostic accuracy of virtual non-contrast enhanced dual-energy CT for diagnosis of adrenal adenoma: a systematic review and meta-analysis. Eur Radiol 27:4324–4335
Patel BN, Vernuccio F, Meyer M et al (2019) Dual-energy CT material density iodine quantification for distinguishing vascular from nonvascular renal lesions: normalization reduces intermanufacturer threshold variability. AJR Am J Roentgenol 212:366–376
Outwater EK, Bhatia M, Siegelman ES, Burke MA, Mitchell DG (1997) Lipid in renal clear cell carcinoma: detection on opposed-phase gradient-echo MR images. Radiology 205:103–107
Kim JK, Kim SH, Jang YJ (2006) Renal angiomyolipoma with minimal fat: differentiation from other neoplasms at double-echo chemical shift FLASH MR imaging. Radiology 239:174
Karlo CA, Donati OF, Burger IA et al (2013) MR imaging of renal cortical tumours: qualitative and quantitative chemical shift imaging parameters. Eur Radiol 23:1738–1744
Ramamurthy NK, Moosavi B, McInnes MDF, Flood TA, Schieda N (2015) Multiparametric MRI of solid renal masses: pearls and pitfalls. Clin Radiol 70:304–316
Schieda N, Lim RS, Krishna S, McInnes MDF, Flood TA, Thornhill RE (2018) Diagnostic accuracy of unenhanced CT analysis to differentiate low-grade from high-grade chromophobe renal cell carcinoma. AJR Am J Roentgenol 210(5):1079–1087
Galmiche C, Bernhard J-C, Yacoub M, Ravaud A, Grenier N, Cornelis F (2016) Is multiparametric MRI useful for differentiating oncocytomas from chromophobe renal cell carcinomas? AJR Am J Roentgenol 208:343–350
Cornelis F, Tricaud E, Lasserre AS (2014) Routinely performed multiparametric magnetic resonance imaging helps to differentiate common subtypes of renal tumours. Eur Radiol 24:1068
Schieda N, Al Dandan O, Kielar AZ, Flood TA, McInnes MDF, Siegelman ES (2014) Pitfalls of adrenal imaging with chemical shift MRI. Clin Radiol 69:1186–1197
Hodgdon T, McInnes MD, Schieda N, Flood TA, Lamb L, Thornhill RE (2015) Can quantitative CT texture analysis be used to differentiate fat-poor renal angiomyolipoma from renal cell carcinoma on unenhanced CT images? Radiology 276:787
McGahan JP, Sidhar K, Fananapazir G et al (2017) Renal cell carcinoma attenuation values on unenhanced CT: importance of multiple, small region-of-interest measurements. Abdom Radiol (NY) 42:2325–2333
Hur BY, Lee JM, Hyunsik W et al (2014) Quantification of the fat fraction in the liver using dual-energy computed tomography and multimaterial decomposition. J Comput Assist Tomogr 38:845–852
Jhaveri KS, Elmi A, Hosseini-Nik H (2015) Predictive value of chemical-shift MRI in distinguishing clear cell renal cell carcinoma from nonclear cell renal cell carcinoma and minimal-fat angiomyolipoma. AJR Am J Roentgenol 205:W79–86
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Nicola Schieda.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• Retrospective
• Case–control study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 17 kb)
Rights and permissions
About this article
Cite this article
Udare, A., Walker, D., Krishna, S. et al. Characterization of clear cell renal cell carcinoma and other renal tumors: evaluation of dual-energy CT using material-specific iodine and fat imaging. Eur Radiol 30, 2091–2102 (2020). https://doi.org/10.1007/s00330-019-06590-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06590-1