Abstract
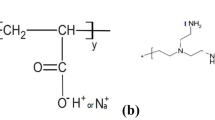
In this work, the structure–property relationships of the thermal gelation of partially hydrolyzed polyacrylamide (PHPA) and polyethylenimine (PEI) mixtures were investigated under realistic conditions of temperature (80 °C) and salinity (total dissolved solids = 3.4 g/l) of the Algerian reservoir (Tin Fouyé Tabankort) prior to a conformance control application. The reactants were characterized with regard to their hydrolysis degree or branching degree using 13C-nuclear magnetic resonance, and viscosity–average molecular weights (\( \bar{M}_{\text{v}} \)) were estimated using the Mark–Houwink equation and intrinsic viscosities measurements. The polymers had molecular weights that varied from 5 to 10 × 106 g/mol for PHPAs with initial hydrolysis degrees between 6 and 20 mol%, while the molecular weights of the PEI were between 2 and 67 × 104 g/mol with a constant branching degree of 57–59. Consequently, the effect of steady shear on the gelation time was investigated followed by the effect of reactant concentrations, the polymer and cross-linker molecular weights, the polymer’s hydrolysis degree, the temperature and the initial pH. All experiments were conducted in a semidilute concentration regime while maintaining practical initial gelant viscosities. As a result, the gelation time was found to decrease with reactant concentrations, molecular weights and temperature (Ea = 62 kJ/mol) and to increase with hydrolysis degree.








Similar content being viewed by others
References
Al-Muntasheri GA (2012) Conformance control with polymer gels: what it takes to be successful. Arab J Sci Eng 37(4):1131–1141
Brattekås B, Fernø MA (2016) New insight from visualization of mobility control for enhanced oil recovery using polymer gels and foams. In: Chemical enhanced oil recovery (cEOR)—a practical overview. InTech
Brattekås B (2014) Conformance control for enhanced oil recovery in fractured reservoirs
Moradi-Araghi A (2000) A review of thermally stable gels for fluid diversion in petroleum production. J Pet Sci Eng 26(1–4):1–10
Abidin A, Puspasari T, Nugroho W (2012) Polymers for enhanced oil recovery technology. Procedia Chem 4:11–16
El-Karsani KSM, Al-Muntasheri GA, Hussein IA (2014) Polymer systems for water shutoff and profile modification: a review over the last decade. SPE J 19(01):135–149
Bai B, Zhou J, Yin M (2015) A comprehensive review of polyacrylamide polymer gels for conformance control. Pet Explor Dev 42(4):525–532
Zhu D, Bai B, Hou J (2017) Polymer gel systems for water management in high-temperature petroleum reservoirs: a chemical review. Energy Fuels 31(12):13063–13087
Broseta D et al (2000) Rheological screening of low-molecular-weight polyacrylamide/chromium (III) acetate water shutoff gels. In: SPE/DOE improved oil recovery symposium. Society of Petroleum Engineers
Sokhanvarian K, Nasr-El-Din HA, Harper TL (2015) Rheological characteristics of CMHPG crosslinked with new zirconium and dual crosslinkers. In: SPE Asia Pacific unconventional resources conference and exhibition. Society of Petroleum Engineers
Han M, Muller G, Zaitoun A (1998) Gel formation of polyacrylamide in the presence of glyoxal for water control application. Polym Gels Netw 5(6):471–480
Yu H et al (2011) Study of a profile control agent applied in an offshore oilfield. Pet Sci Technol 29(12):1285–1297
Reddy B et al (2003) A natural polymer-based cross-linker system for conformance gel systems. SPE J 8(02):99–106
Eoff LS et al (2007) Worldwide field applications of a polymeric gel system for conformance applications. SPE Prod Oper 22(02):231–235
Ghriga MA et al (2019) Review of recent advances in polyethylenimine crosslinked polymer gels used for conformance control applications. Polym Bull. https://doi.org/10.1007/s00289-019-02687-1
Reddy B, Crespo F, Eoff LS (2012) Water shutoff at ultralow temperatures using organically crosslinked polymer gels. In: SPE improved oil recovery symposium. Society of Petroleum Engineers
Vasquez J et al (2005) Development and evaluation of high-temperature conformance polymer systems. In: SPE international symposium on oilfield chemistry. Society of Petroleum Engineers
Morgan J, Smith P, Stevens D (1997) Chemical adaptation and development strategies for water and gas shutoff gels. In: RSC chemistry in the oil industry, 6th international symposium, Charllotte Mason College Ambleside, UK
Al-Muntasheri GA, Nasr-El-Din HA, Zitha PL (2008) Gelation kinetics and performance evaluation of an organically crosslinked gel at high temperature and pressure. SPE J 13(03):337–345
Jia H et al (2010) Research on the gelation performance of low toxic PEI cross-linking PHPAM gel systems as water shutoff agents in low temperature reservoirs. Ind Eng Chem Res 49(20):9618–9624
Jia H et al (2012) New insights into the gelation behavior of polyethyleneimine cross-linking partially hydrolyzed polyacrylamide gels. Ind Eng Chem Res 51(38):12155–12166
Bai Y et al (2015) Gelation study on a hydrophobically associating polymer/polyethylenimine gel system for water shut-off treatment. Energy Fuels 29(2):447–458
Vasquez J et al (2003) Laboratory evaluation of high-temperature conformance polymer systems. In SPE production and operations symposium. Society of Petroleum Engineers
Zhu D et al (2019) Evaluation of terpolymer-gel systems crosslinked by polyethylenimine for conformance improvement in high-temperature reservoirs. SPE J. https://doi.org/10.2118/194004-PA
Hashmat M et al (2016) Crosslinked polymeric gels as loss circulation materials: an experimental study. In: SPE Kingdom of Saudi Arabia annual technical symposium and exhibition. Society of Petroleum Engineers
Vasquez JE, Eoff LS (2010) Laboratory development and successful field application of a conformance polymer system for low-, medium-, and high-temperature applications. In: SPE Latin American and Caribbean petroleum engineering conference. Society of Petroleum Engineers
Reddy B et al (2013) Recent advances in organically crosslinked conformance polymer systems. In: SPE international symposium on oilfield chemistry. Society of Petroleum Engineers
Crespo F et al (2014) Development of a polymer gel system for improved sweep efficiency and injection profile modification of IOR/EOR treatments. In: IPTC 2014—International petroleum technology conference
Zhao J-Z et al (2011) Influences of fracture aperture on the water-shutoff performance of polyethyleneimine cross-linking partially hydrolyzed polyacrylamide gels in hydraulic fractured reservoirs. Energy Fuels 25(6):2616–2624
Al-Muntasheri GA et al (2005) Investigation of a high temperature organic water shutoff gel: reaction mechanisms. In: SPE international improved oil recovery conference in Asia Pacific. Society of Petroleum Engineers
Hardy M et al (1999) The first carbonate field application of a new organically crosslinked water shutoff polymer system. In: SPE international symposium on oilfield chemistry. Society of Petroleum Engineers
Al-Muntasheri GA (2008) Polymer gels for water control: NMR and CT scan studies. http://resolver.tudelft.nl/uuid:4cbce4d4-c25d-4801-94f4-4b6e67ff75cb
Brattekås B, Graue A, Seright RS (2015) Low salinity chase waterfloods improve performance of Cr(III)-acetate HPAM gel in fractured cores. In: SPE international symposium on oilfield chemistry. Society of Petroleum Engineers
Dang T et al (2014) The development and optimization of a polymer conformance control technology in mature reservoirs: laboratory experiments versus field scale simulation. Energy Sources Part A Recovery Utilization Environ Effects 36(11):1219–1233
Al-Muntasheri G, Nasr-El-Din H, Zitha P (2006) Gelation kinetics of an organically crosslinked gel at high temperature and pressure. SPE Paper 104071. In: The first international oil conference and exhibition in Mexico, Cancun, Mexico
Sengupta B, Sharma VP, Udayabhanu G (2011) Development and performance of an eco-friendly cross-linked polymer system for water shut-off technique. In: International petroleum technology conference. International Petroleum Technology Conference
Feng Y et al (2002) Hydrophobically associating polyacrylamides and their partially hydrolyzed derivatives prepared by post-modification. 1. Synthesis and characterization. Polymer 43(7):2055–2064
Pierre TS, Geckle M (1985) 13-NMR analysis of branched polyethyleneimine. J Macromol Sci Chem 22(5–7):877–887
Rodriguez L et al Monitoring thermal and mechanical stability of enhanced oil recovery (EOR) acrylamide based polymers (PAM) through intrinsic viscosity (IV) determination using a new capillary rheology technique. In: SPE EOR conference at oil and gas West Asia. 2016. Society of Petroleum Engineers
Taylor KC, Nasr-El-Din HA (1994) Acrylamide copolymers: a review of methods for the determination of concentration and degree of hydrolysis. J Pet Sci Eng 12(1):9–23
Idris SA, Mkhatresh OA, Heatley F (2006) Assignment of 1H NMR spectrum and investigation of oxidative degradation of poly(ethylenimine) using 1H and 13C 1-D and 2-D NMR. Polym Int 55(9):1040–1048
Antonietti L et al (2005) Core–shell-structured highly branched poly(ethylenimine amide) s: synthesis and structure. Macromolecules 38(14):5914–5920
McCarthy K, Burkhardt C, Parazak D (1987) Mark–Houwink–Sakurada constants and dilute solution behavior of heterodisperse poly(acrylamide‐co‐sodium acrylate) in 0.5 M and 1 M NaCl. J Appl Polym Sci 33(5):1699–1714
von Harpe A et al (2000) Characterization of commercially available and synthesized polyethylenimines for gene delivery. J Control Release 69(2):309–322
Zeynali ME, RABIEI A (2002) Alkaline hydrolysis of polyacrylamide and study on poly(acrylamide-co-sodium acrylate) properties
Gales J et al (1994) Equilibrium swelling and syneresis properties of Xanthan gum-Cr(III) gels. SPE Adv Technol Ser 2(02):190–198
Amir Z, Said IM, Jan BM (2019) In situ organically cross-linked polymer gel for high-temperature reservoir conformance control: a review. Polym Adv Technol 30(1):13–39
Mohamed AI et al (2015) DSC investigation of the gelation kinetics of emulsified PAM/PEI system. J Therm Anal Calorim 122(3):1117–1123
Romero-Zeron LB, Hum FM, Kantzas A (2008) Characterization of crosslinked gel kinetics and gel strength by use of NMR. SPE Reserv Eval Eng 11(03):439–453
Sydansk RD (1990) A newly developed chromium (III) gel technology. SPE Reserv Eng 5(03):346–352
Jia H et al (2016) Evaluation of polyacrylamide gels with accelerator ammonium salts for water shutoff in ultralow temperature reservoirs: gelation performance and application recommendations. Petroleum 2(1):90–97
Jayakumar S, Lane RH (2013) Delayed crosslink polymer gel system for water shutoff in conventional and unconventional oil and gas reservoirs. In: SPE international symposium on oilfield chemistry. Society of Petroleum Engineers
Al-Anazi M et al (2011) Laboratory evaluation of organic water shut-off gelling system for carbonate formations. In: SPE European formation damage conference. Society of Petroleum Engineers
Broseta D et al (2000) Shear effects on polyacrylamide/chromium (III) acetate gelation. SPE Reserv Eval Eng 3(03):204–208
Al-Muntasheri GA et al (2010) Water shut-off with polymer gels in a high temperature horizontal gas well: a success story. In: SPE improved oil recovery symposium. Society of Petroleum Engineers
Al-Muntasheri GA, Zitha PL, Nasr-El-Din HA (2010) A new organic gel system for water control: a computed tomography study. SPE J 15(01):197–207
Al-Muntasheri GA, Nasr-El-Din HA, Hussein IA (2007) A rheological investigation of a high temperature organic gel used for water shut-off treatments. J Pet Sci Eng 59(1–2):73–83
Hatzignatiou DG, Giske NH, Stavland A (2016) Polymers and polymer-based gelants for improved oil recovery and water control applications in naturally fractured chalk formations. In: SPE Bergen one day seminar. Society of Petroleum Engineers, Grieghallen, Bergen, p 23
Park IH, Choi E-J (1996) Characterization of branched polyethyleneimine by laser light scattering and viscometry. Polymer 37(2):313–319
Lewandowska K (2007) Comparative studies of rheological properties of polyacrylamide and partially hydrolyzed polyacrylamide solutions. J Appl Polym Sci 103(4):2235–2241
El Karsani KS et al (2014) Impact of salts on polyacrylamide hydrolysis and gelation: new insights. J Appl Polym Sci. https://doi.org/10.1002/app.41185
El-Karsani KS et al (2014) Gelation kinetics of PAM/PEI system. J Therm Anal Calorim 116(3):1409–1415
Jia H, Chen H (2018) Using DSC technique to investigate the non-isothermal gelation kinetics of the multi-crosslinked chromium acetate (Cr3+)-polyethyleneimine (PEI)-polymer gel sealant. J Pet Sci Eng 165:105–113
Zhao G et al (2013) Study on formation of gels formed by polymer and zirconium acetate. J Sol–Gel Sci Technol 65(3):392–398
Sheng J (2010) Modern chemical enhanced oil recovery: theory and practice. Gulf Professional Publishing, Houston
Sydansk RD, Argabright PA (1987) Conformance improvement in a subterranean hydrocarbon-bearing formation using a polymer gel. Google Patents
Acknowledgements
We would like to thank SNF Floerger company—France—for providing us with the polymers especially Mr. Bruno Giovannetti. We would like also to thank Mr. Anthony Laffore for his assistance in the rheological measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghriga, M.A., Gareche, M., Khodja, M. et al. Structure–property relationships of the thermal gelation of partially hydrolyzed polyacrylamide/polyethylenimine mixtures in a semidilute regime. Polym. Bull. 77, 1465–1488 (2020). https://doi.org/10.1007/s00289-019-02817-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-019-02817-9