Abstract
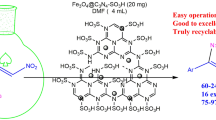
A magnetic nanocomposite of GO-Fe3O4@CuO was fabricated via a simple sonochemical technique and successfully utilized for the ultrasound promoted synthesis of regioselective 1,4-disubstituted mono/bis/tris-1,2,3-triazoles (3a–k). This catalyst could be separated conveniently from the reaction mixture using an external magnet and reused up to eight consecutive runs without noticeable drop in the desired product yield. Other noteworthy features of this green protocol are negligible metal leaching from the support during reaction, high yield in lesser time, aqueous media and good results with gram scale synthesis. Representative compounds were also screened for their cytotoxic activity against PC-12 cell line using standard MTT assay and flow cytometry. Trifluoromethyl group containing triazole derivative (3f) displayed cytotoxic activity (IC50 8.3 µg/mL) comparable to the standard drug cisplatin (IC50 5.8 µg/mL).
Graphic Abstract











Similar content being viewed by others
References
Baig RBN, Varma RS (2013) Chem Commun 49:752–770
Banerjee B (2017) Ultrason Sonochem 35:1–14
Benci K, Mandic L, Suhina T et al (2012) Molecules (Basel, Switzerland) 17:11010–11025
Breslow R (1991) Acc Chem Res 24:159–164
Chetia M, Ali AA, Bhuyan D et al (2015) New J Chem 39:5902–5907
Chiplunkar PP, Zhao X, Tomke PD et al (2018) Ultrason Sonochem 40:587–593
Cravotto G, Fokin VV, Garella D et al (2010) J Comb Chem 12:13–15
Dabiri M, Kasmaei M, Salari P et al (2016) RSC Adv 6:57019–57023
Dehghanzad B, Razavi Aghjeh MK, Rafeie O et al (2016) RSC Adv 6:3578–3585
Dubey N, Sharma P, Kumar A (2015) Synth Commun 45:2608–2626
Fan Q, Lan Q, Zhang M et al (2016) J Semicond 37:083003
Gholinejad M, Jeddi N (2014) ACS Sustain Chem Eng 2:2658–2665
Ghosh D, Rhodes S, Hawkins K et al (2015) New J Chem 39:295–303
Gonzalez MDC, Oton F, Espinosa A et al (2015) Org Biomol Chem 13:1429–1438
Gupta R, Jain A, Jain M et al (2018) Cattle Lett 8:2180–2194
He XP, Zang Y, James TD et al (2016) Chem Commun (Camb) 53:82–90
Hein JE, Fokin VV (2010) Chem Soc Rev 39:1302–1315
Hsu Y-W, Hsu T-K, Sun C-L et al (2012) Electrochimica Acta 82:152–157
Hudson R, Li C-J, Moores A (2012) Green Chem 14:622
Huisgen R (1963) Angew Chem Int Ed 2:565–598
Hummers WS, Offeman RE (1958) J Am Chem Soc 80:1339
Jain A, Jain Y, Gupta R et al (2018) J Fluor Chem 212:153–160
Jain Y, Gupta R, Yadav P et al (2019) ACS Omega 4:3582–3592
Jain Y, Kumari M, Gupta R (2019) Tetrahedron Lett 60:1215–1220
Jiao Y, Zhu B, Chen J et al (2015) Theranostics 5:173–187
Klein R, Brown D, Turnley AM (2007) BMC Neurosci 8:61
Kolb HC, Finn MG, Sharpless KB (2001) Angew Chem Int Ed 40:2004–2021
Kolb HC, Sharpless KB (2003) Drug Discov Today 8:1128–1137
Kumari M, Gupta R, Jain Y (2019) Synth Commun 49:529–538
Liao K-H, Mittal A, Bose S et al (2011) ACS Nano 5:1253–1258
Liu H, Wu X, Li X et al (2014) Chin J Catal 35:1997–2005
Liu S, Xu Q, Latthe SS et al (2015) RSC Adv 5:68293–68298
Lonkar SP, Ahmed AA (2014) J Thermodyn Catal 5:1–6
López-Rojas P, Janeczko M, Kubiński K et al (2018) Molecules 23:199
Naeimi H, Shaabani R (2017) Ultrason Sonochem 34:246–254
Neeru S, Vikas S, Yachana J et al (2017) Macromol Symp 376:1700006
Panaka S, Trivedi R, Jaipal K et al (2016) J Organomet Chem 813:125–130
Petrova KT, Potewar TM, Correia-Da-Silva P et al (2015) Carbohydr Res 417:66–71
Rajaganesh R, Ravinder P, Subramanian V et al (2011) Carbohydr Res 346:2327–2336
Rostovtsev VV, Green LG, Fokin VV et al (2002) Angew Chem Int Ed 41:2596–2599
Saadatjoo N, Golshekan M, Shariati S et al (2017) Arab J Chem 10:S735–S741
Sabaqian S, Nemati F, Heravi MM et al (2017) Appl Organomet Chem 31:e3660
Salamatmanesh A, Kazemi M, Yazdani E et al (2018) Catal Lett 148(10):3257–3268
Sharghi H, Khalifeh R, Doroodmand MM (2009) Adv Synth Catal 351:207–218
Sharma RK, Mishra M, Sharma S et al (2016) J Coord Chem 69:1152–1165
Shaygan Nia A, Rana S, Dohler D et al (2015) Chemistry 21:10763–10770
Shokoohinia Y, Khajouei S, Ahmadi F et al (2017) Iran J Basic. Med Sci 20:438–445
Singh R, Jain S, Poduri R (2013) J Nanoparticle Res 15:1985
Souza JF, Costa GP, Luque R et al (2019) Catal. Sci Technol 9:136–145
Tornøe CW, Christensen C, Meldal M (2002) J Org Chem 67:3057–3064
Vats T, Gogoi R, Gaur P et al (2017) ACS Sustain Chem Eng 5:7632–7641
Wang C, Wang D, Yu S et al (2016) ACS Catal 6:5424–5431
Wang Q, Chan TR, Hilgraf R et al (2003) J Am Chem Soc 125:3192–3193
Wu K, Jing C, Zhang J et al (2019) Appl Surf Sci 466:746–756
Wu P, Feldman AK, Nugent AK et al (2004) Angew Chem (Int Ed Engl) 43:3928–3932
Zaaba NI, Foo KL, Hashim U et al (2017) Procedia Eng 184:469–477
Zhang X, Zhou J, Song H et al (2014) ACS Appl Mater Interfaces 6:17236–17244
Zhu J, Zeng G, Nie F et al (2010) NanoScale 2:988–994
Acknowledgements
Authors are thankful to Materials Research Centre, MNIT Jaipur for providing spectral facilities. Y. Jain and M. Kumari are acknowledges to MNIT Jaipur for providing financial assistance and DST, New Delhi for awarding INSPIRE fellowship scheme (IF140506) respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jain, Y., Kumari, M., Singh, R.P. et al. Sonochemical Decoration of Graphene Oxide with Magnetic Fe3O4@CuO Nanocomposite for Efficient Click Synthesis of Coumarin-Sugar Based Bioconjugates and Their Cytotoxic Activity. Catal Lett 150, 1142–1154 (2020). https://doi.org/10.1007/s10562-019-02982-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02982-6