Abstract
Solid-state 29Si and 71Ga NMR was used to study the synthetic gallosilicate Na16Ga16Si24O80 ·16H2O (Ga–natrolite). It has been shown that Ga–natrolite contains mainly Si(GaO4)3(SiO4) and Si(GaO4)2(SiO4)2-structural units and has sufficiently ordered structure. Temperature dependence of the spin–lattice relaxation time T1 of 71Ga nuclei has also been studied using solid-state NMR. Spin–lattice relaxation of the 71Ga was determined to be governed by the electric quadrupole interaction with the crystal electric field gradients modulated by translational motion of H2O molecules in the Ga–natrolite pores.
Similar content being viewed by others
1 Introduction
Nowadays, there has been considerable interest in the isomorphous replacement of Al atoms by other trivalent cations due to the increased interest in zeolites. These micro-porous species possess different physical and chemical properties from their aluminium analogues and offer possibilities for new applications of molecular sieves [1,2,3,4,5]. Gallium is directly below aluminium in the periodic table; therefore, it forms chemically similar, analogous tetrahedra to AlO4. Isomorphous substitution of Ga for Al in alumosilicate natrolite (Na16Al16Si24O80·16H2O) gives gallosilicate natrolite (Ga–natrolite) [1].
NMR spectroscopy is one of the main methods for studying the microstructure of materials, including zeolites [6]. In present paper, the structure of orthorhombic synthetic gallosilicate natrolite (Na16Ga16Si24O80·16H2O) was studied by means of NMR MAS of 29Si and 71Ga nuclei. The temperature dependence of the spin–lattice relaxation time T1 of 71Ga nuclei has also been studied using solid-state NMR. Obtained results were compared with available references [7,8,9,10,11,12,13,14,15].
2 Experimental Procedure
Gallium form of natrolite was hydrothermally synthesized as described in [12]. Water solution of gallium oxide (Ga2O3, 99.99 + %, Aldrich) and sodium hydroxide (50% aqueous solution, Aldrich) was heated up to 100 °C and stirred over night. After cooling to room temperature, colloidal silica (Ludox AS-40) was slowly added, with stirring. Result mixture with oxide composition of 6.0Na2O·1.0Ga2O3·10.0SiO2·150H2O was charged into Teflon-lined 100 mL autoclave and heated up to 170 °C. After 12 days, the autoclave was cooled down. Products were washed three times with water and dried over night in the oven.
Powder X-ray data (XRD) were collected on PANalytical XPERT-PRO diffractometer using Cu–Kα radiation source (λ = 1.541874 Å). Samples were analyzed over the 2θ range of 5–70° with the step size of 0.017° (Fig. 1). Phases were identified by comparing diffraction peaks with the data reported in Inorganic Crystal Structure Database (ICSD) using X’Pert HighScore program. According to ICSD, obtained XRD pattern matches NAT topology and it is in a good agreement with material TNU-4 as in [12]. This suggests that experimental material is gallium form of natrolite.
Chemical analyses of synthesized gallium natrolite and natural natrolite were performed using DSM 982 Gemini FE-SEM scanning electron microscope (SEM) ZEISS. The detector consisted of the inlens and lateral electron (SE) detector and the backscatter electron (BSE) detector. BSE detector was used most frequently for individual SEM images. Respective excitation voltage was 0.265 keV. Details of chemical analyses can be found in Table 1. SEM picture of small crystals produced by hydrothermal synthesis can be found in Fig. 2.
NMR MAS spectra were obtained on polycrystalline sample of Ga–natrolite on a Bruker Avance-400 NMR spectrometer. 29Si MAS NMR spectra were measured at a spinning rate of 10 kHz using 4 mm rotors at a 29Si frequency of 79.490 MHz with π/2 pulse length of 3 μs. Typically, 10,000 scans were accumulated and the 29Si chemical shifts were referenced to TMS. 71Ga MAS NMR spectra were obtained at a 71Ga frequency of 122.0564 MHz in 4 mm rotors at a spinning rate of 14.0 kHz with an acquisition of 1000 pulse transients, which was repeated with RF pulse length of 2.4 μs. The Dmfit program [16] was used to simulate 71Ga spectra to extract isotropic chemical shifts (\(\delta_{iso}\)), quadrupolar coupling constants (CQ), and asymmetry parameters (η). The spin–lattice relaxation time T1 for 71Ga nuclei was measured by the saturation-recovery method.
3 Results and Discussion
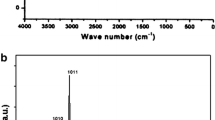
Figure 3 shows 29Si MAS NMR spectra of Al–and Ga–natrolites. From these figure, it follows that 29Si MAS NMR spectra of Al– and Ga–natrolite are similar and contain two resonant lines. In natural Al–natrolite, two resonance lines of 29Si MAS NMR spectrum at– 84.9 ppm and – 92.7 ppm with an intensity ratio of ≈ 2:1 were observed in our investigations (Fig. 3).
29Si MAS NMR spectra of Al–natrolite [13] (a) and Ga–natrolite (b)
The number of resonance NMR lines of the nuclei 29Si and 71Ga determined by the degree of ordered of the natrolite structure [6, 14, 17]. It has been demonstrated at first by Lipmaa et al. [17] that chemical shifts of 29Si MAS NMR spectra of zeolite and other aluminoosilicates are sensitive to the number of AlO4 tetrahedra linked to the SiO4 tetrahedron, also described as the second coordination sphere of silicon. The number of aluminium tetrahedra sharing oxygens with the SiO4 tetrahedron defines five different structural units in the alumosilicate framework, namely, Si(nAl), where index n = 0, 1, 2, 3, 4 specify the number of aluminium tetrahedra connected with SiO4 tetrahedron. 29Si chemical shift ranges by about 5 ppm low-field shifts for each additional connected substituent [17]. This allows to establish structures of various zeolites and get information about Si/Al ordering.
The orthorhombic Al–natrolite (space group Fdd2) has three different central sites (T-sites) in TO4 tetrahedra with multiplicities 8:16:16. According to the X-ray and NMR structure determination study [17, 18], the structure of natural Al–natrolite is well-ordered with Si/Al ratio of 3:2 [6, 17]. There are two types of species with silicon atoms containing Si/Al ratio of 1.5, i.e., those connected to three AlO4 tetrahedra and one SiO4 tetrahedron (Si(AlO4)3(SiO4)) and those connected to two AlO4 tetrahedra and two SiO4 tetrahedra (Si(AlO4)2(SiO4)2) in 2:1 ratio [17].
Two resonance lines at – 81.1 ppm and – 91.1 ppm observed in NMR MAS 29Si spectrum (Fig. 3) of gallosilicate natrolite may indicate that the natrolite has sufficiently well-ordered structure and mainly contains Si(GaO4)3(SiO4) and Si(GaO4)2(SiO4)2) structural units. However, the intensity ratio of these two lines (≈ 1.7) is somewhat unusual and has an Si/Ga ratio different than 1.5. This suggests that in the structure of gallosilicate natrolite, there are other Si(GaO4)n(SiO4)4 − n species with n = 0, 1, 4 structural units [11]. Intensities of NMR MAS 29Si lines of these units may be too weak to be observed experimentally [11]. The difference of chemical shifts between two resonance lines of 29Si atoms in Ga–natrolite is 10.0 ppm.
Gallium atoms have four nearest-neighbour oxygen atoms in tetrahedral coordination in Ga–natrolite. The nucleus of gallium atom has the spin of I = 3/2 and it is a quadrupolar nucleus. The quadrupole moment of the nucleus is a sensitive probe of the existence of a nonuniform electric field (EFG) at the location of the nucleus. The experimental and simulated 71Ga MAS NMR spectra of Ga–natrolite are shown in Fig. 4 which clearly shows a line shape that is determined by the second-order quadrupolar interaction [19, 20].
The experimental temperature dependence of the spin–lattice relaxation time T1 of 71Ga nuclei in Ga–natrolite is shown in Fig. 5. It is important to note that the relaxation process for 71Ga nuclei in Ga–natrolite was well described by a single exponential. It can be assumed that the main physical mechanism responsible for spin–lattice relaxation of 71Ga nuclei in Ga–natrolite is identical with the mechanism of spin–lattice relaxation of 27Al nuclei in Al–natrolite and the spin–lattice relaxation of 71Ga nuclei in Ga–natrolite is concerned with modulation of electric quadrupolar interactions at the sites of the 71Ga nuclei by thermal motion of water molecules [14]. In this case, the rate of spin–lattice relaxation of 71Ga nuclei may be described by the equation as [14, 20,21,22,23]
where parameter α depends on the asymmetry parameter η of the EFG (from the estimations α ≈ 0.13–0.17 [23]); ω0 is the Larmor frequency of the quadrupole nucleus and
describes fluctuations of the quadrupole coupling constant [20] at the sites of the 71Ga nuclei, \(\tau_{\text{c}}\) is the correlation time, which describes activated translational and reorientational jumps of electric dipoles in water molecules. In Eq. (1), \(\overline{\left( \cdots \right)}\) represents an ensemble average.
From Eq. (1) ,it follows that \(R_{{1{\text{Q}}}} \sim \tau_{\text{c}}\) for \(\omega_{0} \tau_{\text{c}} < < 1\), while \(R_{{1{\text{Q}}}} \sim 1/\tau_{\text{c}}\) for \(\omega_{0} \tau_{\text{c}} > > 1\), with a maximum of \(R_{{1{\text{Q}}}}\) (and minimum of \(T_{{1{\text{Q}}}}\)) in the intermediate region. We assume that the correlation time \(\tau_{\text{c}}\) caused by the molecular motion follows the Arrhenius-type temperature dependence [20]:
Translational and reorientational jumps of electric dipoles of water molecules were assumed to be dynamically heterogeneous (i.e., described by different activation energies \(E_{\text{a}}\)), and as a result, these were characterized by a normal distribution of the activation energies [24]:
Therefore, Eq. (1) was averaged over distribution function of Eq. (4). Experimental temperature dependence of the spin–lattice relaxation time and results of their approximation by function of Eq. (1) are presented in Fig. 5. Obtained adjusting parameters are given in Table 2.
Obtained activation energy 7.3 kcal/mol strongly suggests that translational and reorientational jumps of electric dipoles of water molecules are responsible for the relaxation process of the 71Ga nuclei.
According to the data presented in Table 1, averaged fluctuations of quadrupole coupling constant at the sites of the 71Ga nuclei are
Full constant of quadrupolar interaction for the 71Ga nuclei in the natrolite \(C_{\text{Q}} = {\text{eqQ}}/h = 5\;{\text{MHz}}\), and therefore, the contribution of the electric dipolar moments of the water molecules to the full EFG at the 71Ga sites is 6%.
4 Conclusions
Based on the analysis of obtained NMR MAS spectra of 29Si and 71Ga nuclei in Ga–natrolites, synthetic gallosilicate natrolite has a well-ordered structure with Si/Ga ratio (3:2) and it contains (Si(GaO4)3(SiO4)) and (Si(GaO4)2(SiO4)2) tetrahedra in the 2:1 ratio. From experimental and simulated 71Ga MAS NMR spectrum of Ga–natrolite, it follows that NMR line shape is determined by the second-order quadrupolar interaction. The spin–lattice relaxation of the 71Ga is governed by electric quadrupole interaction with crystal electric field gradients modulated by translational motion of H2O molecules in natrolite pores.
References
D.W. Breck, Zeolite Molecular Sieves (Wiley, New York, 1974)
J. Weitkamp, in Catalysis and Absorption by Zeolites, ed. by G. Olhmann, J.C. Vedrine, P.A. Jacobs (Elsevier, Amsterdam, Netherland, 1991), p. 21
J.M. Newsam, in Solid State Chemistry Compounds, ed. by A.K. Cheetham, P. Day (Oxford University Press, Oxford, 1992), pp. 234–280
S.M. Auerbach, H.I. Metiu, J. Chem. Phys. 106(7), 2893 (1997)
J.B. Nagy, R. Aiello, G. Giordano, A. Katovic, F. Testa, Z. Kónya, I. Kiricsi, Mol. Sieves 5, 365 (2007)
G. Engelhardt, D. Michel, High-Resolution Solid-State NMR of Silicates and Zeolites (Wiley, New York, 1987), p. 150
S.B. Hong, S.H. Kim, Y.G. Kim, Y.C. Kim, P.A. Barrett, M.A. Camblor, J. Mater. Chem. 9, 2287 (1999)
S.B. Hong, S.H. Lee, C.-H. Shin, A.J. Woo, J.L. Alvarez, M.C. Zicovich-Wilson, M.A. Camblor, J. Am. Chem. Soc. 126(42), 13742 (2004)
A. V. Sapiga, Thesis, Tavrida National University at the name of V.I.Vernadsky, Simferopol, Ukraine, 2003
M. Paczwa, Thesis, Department of Mathematics and Physics, Institute of Physics, University of Szczecin, Poland, 2017
H.K.C. Timken, E. Oldfield, J. Am. Chem. Soc. 109, 7669 (1987)
H.H. Cho, S.H. Kim, Y.G. Kim, Y.C. Kim, H. Koller, M.A. Camblor, S.B. Hong, Chem. Mater. 12, 2292 (2000)
A.A. Sapiga, M. Olszewski, M. Paczwa, A.V. Sapiga, N.A. Sergeev, Funct. Mater. 21, 181 (2014)
M. Paczwa, A.A. Sapiga, M. Olszewski, N.A. Sergeev, A.V. Sapiga, Appl. Magn. Reson. 46, 583 (2015)
A.M. Panich, I.A. Belitskii, N.K. Moroz, S.P. Gabuda, V.A. Drebushchak, Yu.V. Seretkin, J. Struct. Chem. 31, 56 (1990)
D. Massiot, F. Fayon, M. Capron, I. King, S. Calve, B. Alonso, J.-O. Durand, B. Bujoli, Z. Gan, G. Hoatson, Magn. Reson. Chem. 40, 70 (2002)
E. Lippmaa, M. Magi, A. Samoson, M. Tarmak, G. Engelhardt, J. Am. Chem. Soc. 103, 4992 (1981)
W.M. Meier, Z. Kristallogr. 113, 430 (1960)
D. Freude, in Encyclopedia of Analytical Chemistry, ed. by R.A. Meyers (Wiley, Chichester, 2000), p. 12188
A. Abragam, The Principles of Nuclear Magnetism (Oxford U.P, London, 1961), Chap. X, p. 467
V.I. Chizhik, Nuclear Magnetic Relaxation (LGU, Leningrad, 1991), p. 99, Eq. (3.111)
J. Haase, H. Pfiefer, W. Oehme, J. Klinowski, Chem. Phys. Lett. 150, 189 (1988)
J. Haase, K.D. Park, K. Guo, H.K.C. Timken, E. Oldfield, J. Phys. Chem. 95, 6996 (1991)
A.V. Sapiga, N.A. Sergeev, Cryst. Res. Technol. 36, 8 (2001)
Acknowledgements
We thank Olaf Walter for XRD pattern studies, Philipp Becker for participation in hydrothermal synthesis, Wilhelm Habicht for scanning electron/scanning probe microscopy, from Institute of Technical Chemistry Division of Chemical–Physical Processing (ITC/CPV), Karlsruhe Institute of Technology (KIT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Paczwa, M., Sapiga, A.A., Olszewski, M. et al. NMR Study of Synthetic Gallosilicate Natrolite. Appl Magn Reson 51, 33–40 (2020). https://doi.org/10.1007/s00723-019-01163-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00723-019-01163-3