Abstract
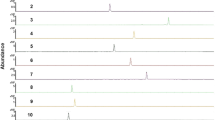
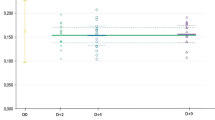
Certified reference materials (CRMs) are the backbone for qualitative and quantitative determination of pesticides in technical materials, formulations, and food matrices. However, their stringent expiry date, limited shelf life, and diminishing purity with time, limits their use beyond expiry. Stability study of 89 CRMs of multiclass pesticides, stored at − 25 °C, has been conducted to assess the purity of CRMs beyond their expiry date. It was evident that > 96 % of the CRMs remained optimally stable with respect to their observed purity even after their expiry date as per certificate of analysis. Percentage deviations in purity of expired and valid CRMs posited well below 7 % and are within ± 10 % acceptable range as recommended by the SANTE. During 2013–2017, performance of the valid and expired CRMs was evaluated through 44 z-scores obtained in 14 international and national proficiency testing programmes. All the z-scores received for 15 expired and 29 valid CRMs were found satisfactory. Inter-laboratory comparison of 6 randomly selected expired and valid CRMs was tested at three different ISO/IEC 17025 accredited laboratories and their % difference between purities ranged from − 2.35 % to + 0.95 %. It was inferred that by maintaining proper storage conditions and continuous monitoring of purity, the expired CRMs can give comparable results as valid CRMs.

Similar content being viewed by others
References
Armishaw P (2016) Certified reference materials—a path to traceable chemical measurements. Department of Industry, Innovation and Science, National Measurement Institute, 5 May 2016, 1–26. https://www.easurement.gov.au. Accessed 24 Oct 2018
BIPM, IEC, IFCC, ILAC, ISO, IUPAC, IUPAP and OIML (2012) International vocabulary of metrology—Basic and general concepts and associated terms (VIM), 3rd edn. JCGM 200:2012. BIPM. https://www.bipm.org/utils/common/documents/jcgm/JCGM_200_2012.pdf. Accessed 25 Sept 2019
ISO/IEC 17025 (2005) General requirements for the competence of calibration and testing laboratories, 2nd edn. International Organization for Standardization (ISO), Geneva, Switzerland
Reenie M (2015) Parris. Panel 11: reference materials and proficiency tests. Certified reference materials and proficiency tests: Provision, production, and value assignment. National Institute of Standards and Technology (NIST), USA. 11 Dec 2015, pp 1–76 http://www.resag.org.br/congressoresag2015/anais/img/pdfs/20160301092315.pdf. Accessed 24 Oct 2018
ISO 17034 (2016) General requirements for the competence of reference material producers. International Organization for Standardization (ISO), Geneva, Switzerland
ISO Guide 30 (2015) Reference materials-selected terms and definitions. International Organization for Standardization (ISO), Geneva, Switzerland
ISO Guide 31 (2015) Reference materials-contents of certificates, labels and accompanying documentation. International Organization for Standardization (ISO), Geneva, Switzerland
ISO Guide 35 (2017) Reference materials-guidance for characterization and assessment of homogeneity and stability. International Organization for Standardization (ISO), Geneva, Switzerland
De Biévre P, Dybkaer R, Fajgelj A, Hibbert DB (2011) Metrological traceability of measurement results in chemistry: concepts and implementation (IUPAC Technical Report). Pure Appl Chem 83(10):1873–1935
Guimarães EF, do Rego ECP, Cunha HCM, Rodrigues JM, Figueroa-Villar JD (2014) Certified reference material for traceability in environmental analysis: pAHs in toluene. J Braz Chem Soc 25(2):351–360
Rettinger M, Sreenivasan U, Dilek I, Pogue S (2010) What makes a good reference standard? Cerilliant analytical reference standard. Cerilliant Corporation, Round Rock, pp 1–32
Pauwels J, Lamberty A, Schimmel H (1998) Quantification of the expected shelf-life of certified reference materials. Fresenius’ J Anal Chem 361(5):395–399
Lamberty A, Schimmel H, Pauwels J (1998) The study of the stability of reference materials by isochronous measurements. Fresenius’ J Anal Chem 360:359–361
SANTE (2017) Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed. SANTE/11813/2017, 21–22 November 2017 rev.0, European Commission Directorate General for Health and Food Safety, Bruxelles/Brussel, Belgium, pp 1–46
WHO (2006a) WHO standardization. Recommendations for the preparation, characterization and establishment of international and other biological reference standards (revised 2004). WHO technical report series 2006, no. 932
WHO (2006b) WHO working group on stability of reference materials for biological medicines and in vitro diagnostics. Geneva, Switzerland, 27–28 November 2006, pp 1–15
Tahlan A, Barrowcliffe T, Das RG, Shin J, Wood D (2005) WHO technical workshop on stability of reference materials for biological medicines and in vitro diagnostics, Geneva, Switzerland, 28–29 November 2005. Biologicals 2007
Thompson M (2016) Analytical methods committee, AMCTB no. 74: z-scores and other scores in chemical proficiency testing-their meanings, and some common misconceptions. Anal Methods 8:5553–5555
ISO 13528 (2015) Statistical methods for use in proficiency testing by inter-laboratory comparison. International Organization for Standardization (ISO), Geneva
Cunningham WC, Capar SG (2014) Elemental analysis manual for food and related products. Elemental analysis manual (Section 3.5 reference materials). FDA, US Food and Drug Administration, US Department of Health and Human Services, USA. 1–9 http://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm2006954.html. Accessed 24 Oct 2018
Roelandts I, Gladney ES (1998) Consensus values for NIST biological and environmental standard reference materials. Fresenius’ J Anal Chem 360:327–338
ISO Guide 34 (2009) General requirements for the competence of reference material producers. International Organization for Standardization (ISO), Geneva
ISO/IEC 17043 (2010) Conformity assessment—general requirements for proficiency testing. International Organization for Standardization (ISO), Geneva
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) Fast and easy multi-residue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J AOAC Int 86:412–431
Wu J, Lu J, Wilson C, Lin Y, Lu H (2010) Effective liquid-liquid extraction method for analysis of pyrethroid and phenylpyrazole pesticides in emulsion-prone surface water samples. J Chromatogr A 1217(41):6327–6333
National Measurement Institute (NMI) (2016) Proficiency test report AQA 15–16 Cocaine. January 2016. National Measurement Institute, Department of Industry, Innovation and Science, Australian Government, pp 1–30
Acknowledgements
This work was supported by ICAR—Indian Council of Agricultural Research and Department of Agriculture, Cooperation and Farmers Welfare, Ministry of Agriculture, Government of India, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, K.K., Tripathy, V., Gautam, R. et al. Monitoring of purity and stability of CRMs of multiclass pesticides during prolonged storage before and after expiration. Accred Qual Assur 25, 89–97 (2020). https://doi.org/10.1007/s00769-019-01411-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00769-019-01411-w