Abstract
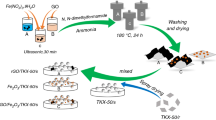
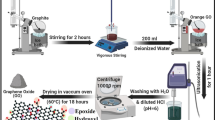
Fe3O4/reduced graphene oxide (Fe3O4/rGO) nanocomposite has been successfully fabricated using a modified interface solvothermal method and characterized by X-ray diffraction, Raman spectroscopy, Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy. In the preparation procedure, graphene oxide was reduced and ultrafine Fe3O4 nanoparticles (NPs) were uniformly loaded on reduced graphene oxide (rGO) carrier. Scanning electron microscope, transmission electron microscope images and Brunauer–Emmett–Teller specific surface area revealed that the aggregation of Fe3O4 NPs was greatly reduced by introducing rGO as a substrate. The average size of the Fe3O4 NPs anchored on the graphene sheets was 100 nm, which is much smaller than 1-μm bare Fe3O4. The DSC results showed that Fe3O4/rGO nanocomposite reduces the first decomposition temperature of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50) by 44.9 °C and decreases the apparent activation energy of TKX-50 by 26.2 kJ mol−1, which exhibits higher catalytic performance than its individual components and their physical mixture (hybrid). Hence, Fe3O4/rGO nanocomposite can be a promising additive for insensitive solid propellants based on TKX-50.






Similar content being viewed by others
References
Venugopalan S. Demystifying explosives-concepts in high energy materials. Amsterdam: Elsevier; 2015.
Song ZW, Yan QL, Li XJ, Qi XF, Liu M. Crystal transition of ε-CL-20 in different solvent. Chin J Energ Mater. 2010;18:648–53.
Golovina NI, Utenyshev AN, Bozhenko KV, Chukanov NV, Zakharov VV, Korsounskii BL. The energy parameters of 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazaisowurtzitane polymorphs and their phase transitions. Russ J Phys Chem A. 2009;83:1153–9.
Fischer N, Fischer D, Klapoetke TM, Piercey DG, Stierstorfer J. Pushing the limits of energetic materials—the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate. J Mater Chem. 2012;22:20418–22.
Sinditskii VP, Filatov SA, Kolesov VI, Kapranov KO, Asachenko AF, Nechaev MS, Lunin VV, Shishov NI. Combustion behavior and physico-chemical properties of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate (TKX-50). Thermochim Acta. 2015;614:85–92.
Bi FQ, Fu XL, Shao ZB, Fan XZ, Li JZ, Yu HJ. Calculation of energy characteristics of high energy monopropellant TKX-50. Chem Propell Polym Mater. 2013;5:70–3.
Wang XP, Luo YJ, Guo K, Lu Y. Energy characteristics computation of propellant containing 3,3′-Dinitro-4,4′-oxazafurazan. Chin J Energ Mater. 2009;17:79–82.
Klapötke TM, Witkowski TG, Wilk Z, Hadzik J. Determination of the initiating capability of detonators containing TKX-50, MAD-X1, PETNC, DAAF, RDX, HMX or PETN as a base charge, by underwater explosion test. Prop Explos Pyrotech. 2016;41:92–7.
Lurnan JR, Wehrman B, Kuo KK, Yetter RA, Masoud NM, Manning TG, Harris LE, Bruck HA. Development and characterization of high performance solid propellants containing nano-sized energetic ingredients. Proc Combust Inst. 2007;31:2089–96.
Sinditskii VP, Egorshev VY, Serushkin VV, Levshenkov AI, Berezin MV, Filatov SA, Smirnov SP. Evaluation of decomposition kinetics of energetic materials in the combustion wave. Thermochim Acta. 2009;496:1–12.
Korobeinichev OP, Paletskii AA, Volkov EN. Flame structure and combustion chemistry of energetic materials. Russ J Phys Chem B. 2008;2:206–28.
Xie MZ, Heng SY, Liu ZR, Wang H, Wang XH, Zhao FQ. Research on the catalytic thermal decomposition of RDX-CMDB propellants by TG-DSC-IR-MS. J Solid Rock Tech. 2009;32:539–42.
Huang HF, Shi YM, Yang J. Thermal characterization of the promising energetic material TKX-50. J Therm Anal Calorim. 2015;121:705–9.
An Q, Liu WG, Goddard WA, Cheng T, Zybin SV, Xiao H. Initial steps of thermal decomposition of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate crystals from quantum mechanics. J Phys Chem C. 2014;118:27175–81.
Wang JF, Chen SS, Jin SH, Shi R, Yu ZF, Su Q, Ma X, Zhang CY, Shu QH. The primary decomposition product of TKX-50 under adiabatic condition and its thermal decomposition. J Therm Anal Calorim. 2018;134:2049–55.
Wang JF, Chen SS, Jin SH, Shu QH, Zhang XP, Shi R. Thermal behavior, compatibility study and safety assessment of diammonium 5,50-bistetrazole-1,10-diolate (ABTOX). J Therm Anal Calorim. 2019. https://doi.org/10.1007/s10973-019-08605-x.
Qin H, Zha M, Ma Z, Zhao F, Xu S, Xu H. Controllable fabrication of CuO/ammonium perchlorate (AP) nanocomposites through ceramic membrane anti-solvent recrystallization. Prop Explos Pyrotech. 2014;39:694–700.
Zhang Y, Wei T, Xu KZ, Ren Z, Xiao L, Song J, Zhao FQ. Catalytic decomposition action of hollow CuFe2O4 nanospheres on RDX and FOX-7. RSC Adv. 2015;5:75630–5.
Gao HX, Zhao FQ, Luo Y, Hao HX, Pei Q, Li SW. Synthesis of nanocomposite PbO·SnO2 and its effect on the combustion properties of DB and RDX-CMDB propellants. Chin J Expl Propell. 2012;35:15–8.
Zhang Y, Xiao L, Xu KZ, Song J, Zhao FQ. Graphene oxide-enveloped Bi2WO6 composites as a highly efficient catalyst for the thermal decomposition of cyclotrimethylene -trinitramine. RSC Adv. 2016;6:42428–34.
Yan W, Cao X, Ke K, Tian J, Jin C, Yang R. One-pot synthesis of monodispersed porous CoFe2O4 nanospheres on graphene as an efficient electrocatalyst for oxygen reduction and evolution reactions. RSC Adv. 2016;6:307–13.
Zu YQ, Zhang Y, Xu KZ, Zhao FQ. Graphene oxide-MgWO4 nanocomposite as an efficient catalyst for the thermal decomposition of RDX, HMX. Rsc Adv. 2016;6:31046–52.
Eigler S, Hirsch A. Chemistry with graphene and graphene oxide-challenges for synthetic chemists. Angew Chem Int Edit. 2014;53:7720–38.
Huang X, Qi X, Boey F, Zhang H. Graphene-based composites. Chem Soc Rev. 2012;41:666–86.
Salamon J, Sathishkumar Y, Ramachandran K, Lee YS, Yoo DJ, Kim AR, Kumar GG. One-pot synthesis of magnetite nanorods/graphene composites and its catalytic activity toward electrochemical detection of dopamine. Biosens Bioelectron. 2015;64:269–76.
Vinothkanna M, Karthikeyan C, Kumar GG, Kim AR, Yoo DJ. One-pot green synthesis of reduced graphene oxide (RGO)/Fe3O4 nanocomposites and its catalytic activity toward methylene blue dye degradation. Spectrochim Acta A. 2015;136:256–64.
Rani GJ, Babu KJ, Kumar GG, Rajan MAJ. Watsonia meriana flower like Fe3O4/reduced graphene oxide nanocomposite for the highly sensitive and selective electrochemical sensing of dopamine. J Alloy Compd. 2016;688:500–12.
Cui ZM, Jiang LY, Song WG, Guo YG. High-yield gas-liquid interfacial synthesis of highly dispersed Fe3O4 nanocrystals and their application in lithium-ion batteries. Chem Mater. 2009;21:1162–6.
Chandra V, Park J, Chun Y, Lee JW, Hwang I, Kim KS. Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano. 2010;4:3979–86.
Shi R, Yan L, Xu T, Liu D, Zhu Y, Zhou J. Graphene oxide bound silica for solid-phase extraction of 14 polycyclic aromatic hydrocarbons in mainstream cigarette smoke. J Chromatogr A. 2015;1375:1–7.
Zhou G, Wang D, Yin L, Li N, Li F, Cheng H. Oxygen bridges between NiO nanosheets and graphene for improvement of lithium storage. ACS Nano. 2012;6:3214–23.
Chen T, Du P, Jiang W, Liu J, Hao GZ, Gao H, Xiao L, Ke X, Zhao FQ, Xuan CL. A facile one-pot solvothermal synthesis of CoFe2O4/RGO and its excellent catalytic activity on thermal decomposition of ammonium perchlorate. RSC Adv. 2016;6:83838–47.
Zaitsev VS, Filimonov DS, Presnyakov IA, Gambino RJ, Chu B. Physical and chemical properties of magnetite and magnetite-polymer nanoparticles and their colloidal dispersions. J Colloid Interf Sci. 1999;212:49–57.
Ganguly A, Sharma S, Papakonstantinou P, Hamilton J. Probing the thermal deoxygenation of graphene oxide using high-resolution in situ X-ray-based spectroscopies. J Phys Chem C. 2011;115:17009–19.
Petit C, Seredych M, Bandosz TJ. Revisiting the chemistry of graphite oxides and its effect on ammonia adsorption. J Mater Chem. 2009;19:9176–85.
Thiruvengadathan R, Chung SW, Basuray S, Balasubramanian B, Staley CS, Gangopadhyay K, Gangopadhyay S. A versatile self-assembly approach toward high performance nanoenergetic composite using functionalized graphene. Langmuir. 2014;30:6556–64.
Li H, Xu T, Wang C, Chen J, Zhou H, Liu H. Tribochemical effects on the friction and wear behaviors of diamond-like carbon film under high relative humidity condition. Tribol Lett. 2005;19:231–8.
Zhou J, Song H, Ma L, Chen X. Magnetite/graphene nanosheet composites: interfacial interaction and its impact on the durable high-rate performance in lithium-ion batteries. RSC Adv. 2011;1:782–91.
Fujii T, de Groot F, Sawatzky GA, Voogt FC, Hibma T, Okada K. In situ XPS analysis of various iron oxide films grown by NO2-assisted olecular-beam epitaxy. Phys Rev B. 1999;59:3195–202.
Lian P, Zhu X, Xiang H, Li Z, Yang W, Wang H. Enhanced cycling performance of Fe3O4-graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim Acta. 2010;56:834–40.
Fitzgerald RP, Brewster MQ. Flame and surface structure of laminate propellants with coarse and fine ammonium perchlorate. Combust Flame. 2004;136:313–26.
Lu ZP, Xiong Y, Xue XG, Zhang CY. Unusual protonation of the hydroxylammonium cation leading to the low thermal stability of hydroxylammonium-based salts. J Phys Chem C. 2017;121:27874–85.
Kissinger HE. Reaction kinetics on differential thermal analysis. Anal Chem. 1957;29:1702–6.
Yan QL, Zeman S, Zhang JG, Qi XF, Li T, Musil T. Multistep thermolysis mechanisms of azido-s-triazine derivatives and kinetic compensation effects for the rate-limiting processes. J Phys Chem C. 2015;119:14861–72.
Geim AK, Novoselov KS. The rise of graphene. Nat Mater. 2007;6:183–91.
Acknowledgements
The financial assistance from the National Natural Science Foundation of China (21173163, 21473130 and 21503163) is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, J., Zhao, F., Yang, Y. et al. Enhanced catalytic performance on the thermal decomposition of TKX-50 by Fe3O4 nanoparticles highly dispersed on rGO. J Therm Anal Calorim 140, 1759–1767 (2020). https://doi.org/10.1007/s10973-019-08891-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08891-5