Abstract
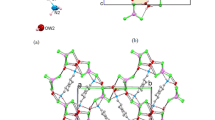
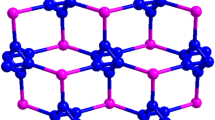
Crystal shapes can be affected by different solvents from sheet, rod, to block because they can enter into the crystal lattice and co-crystallize with the ligands from various instantaneous microenvironments. In this study, two new supramolecular solvates, formulated as [(C8H4NO6+)·(C4H8N5−)]·H2O (1) and [(C8H3NO62+)·(C4H8N5−)2]·CH3OH (2), were synthesized by the combination of 2,4-diamino-6-methyl-1,3,5-triazine (DAMT) and 5-nitroisophthalic acid (H2NIPA) from the solvent mixture of CH3COCH3–H2O/CH3OH–H2O, respectively. Interestingly, the structures of these two solvates contain both classical hydrogen bond N–H⋯O and heterosynthon \({\text{R}}_{2}^{2}\) (8). And due to the different solutions, the supramolecular aggregates led to different crystal structures and shapes. Structural analysis shows that the differences in the two solvate structures mainly attributed to the unique hydrogen bonding pattern between the dimers and solvents molecules. 1 and 2 were characterized by single crystal X-ray diffraction, element analysis, infrared radiation, and thermogravimetric analysis.
Graphical abstract
Two new supramolecular solvates were synthesized by the combination of 2,4-diamino-6-methyl-1,3,5-triazine (DAMT) and 5-nitroisophthalic acid (H2NIPA) from the solvent mixture of CH3COCH3–H2O/CH3OH–H2O.





Similar content being viewed by others
References
Luo YH, Chen C, Hong DL, He XT, Wang JW, Sun BW (2018) J Phys Chem Lett 9:2158–2163
Wang L, Zhao L, Xu LY, Chen RX, Yang Y (2012) CrystEngComm 14:6998–7008
Wang L, Zhao L, Hu YJ, Wang WQ, Chen RX, Yang Y (2013) CrystEngComm 15:2835–2852
Wang L, Zhao L, Liu WM, Chen RX, Gu YX, Yang Y (2012) Sci China Chem 55:2381–2387
Luo YH, Chen C, Hong DL, He XT, Wang JW, Ding T, Wang BJ, Sun BW (2018) ACS Appl Mater Interfaces 10:9495–9502
Han LL, Hu TP, Chen JS, Li ZH, Wang XP, Zhao YQ, Li XY, Sun D (2014) Dalton Trans 43:8774–8780
Vekilov PG (2010) Cryst Growth Des 10:5007–5019
Liu JK, Wu QS, Ding YP (2015) Cryst Growth Des 5:445–449
Han LL, Hu TP, Mei K, Guo ZM, Yin C, Wang YX, Zheng J, Wang XP, Sun D (2015) Dalton Trans 44:6052–6061
Li SB, Ma HY, Pang HJ, Zhang L (2014) Cryst Growth Des 14:4450–4460
Ayoubi MA, Almdal K, Zhu K, Nyström B, Olsson U, Piculell L (2015) RSC Adv 5:31091–31103
Sun D, Li YH, Hao HJ, Liu FJ, Wen YM, Huang RB, Zheng LS (2011) Cryst Growth Des 11:3323–3327
Han LL, Li ZH, Chen JS, Wang XP, Sun D (2014) Cryst Growth Des 14:1221–1226
Danylyuk O, Butkiewicz H, Coleman AW, Suwinska K (2015) CrystEngComm 17:1745–1749
Rager T, Hilfiker R (2010) Cryst Growth Des 10:3237–3241
Das D, Barbour LJ (2009) Cryst Growth Des 9:1599–1604
Sládková V, Skalická T, Skořepová E, Čejka J, Eigner V, Kratochvíl B (2015) CrystEngComm 17:4712–4721
Fleischman SG, Kuduva SS, McMahon JA, Moulton B, Walsh RDB, Hornedo NR, Zaworotko MJ (2003) Cryst Growth Des 3:909–919
Almarsson Ö, Zaworotko MJ (2014) Chem Commun 17:1889–1896
Marjo CE, Bhadbhade M, Hook JM, Rich AM (2011) Mol Pharmaceutics 8:2454–2464
Almarsson Ö, Hickey MB, Peterson ML, Morissette SL, Soukasene S, McNulty C, Tawa M, MacPhee JM, Remenar JF (2003) Cryst Growth Des 3:927–933
Clarke HD, Arora KK, Bass H, Kavuru P, Ong TT, Pujari T, Wojtas L, Zaworotko MJ (2010) Cryst Growth Des 10:2152–2167
Takieddin K, Khimyak YZ, Fábián L (2016) Cryst Growth Des 16:70–81
Day J, Marriott KER, Kilner CA, Halcrow MA (2014) New J Chem 34:52–60
Patil RS, Drachnik AM, Kumari H, Barnes CL, Deakyne CA, Atwood JL (2015) Cryst Growth Des 15:2781–2786
Suzuki M, Kobayashi K (2011) Cryst Growth Des 11:1814–1820
Iwata K, Kojima T, Ikeda Y (2014) Cryst Growth Des 1:3335–3342
Li YT, Li L, Zhu YP, Meng XG, Wu AX (2009) Cryst Growth Des 9:4255–4257
Chen JX, Wang JK, Ulrich J, Yin QX, Xue LZ (2008) Cryst Growth Des 8:1490–1494
Chakravarty P, Suryanarayanan R (2010) Cryst Growth Des 10:4414–4420
Ouhib F, Raynal M, Jouvelet B, Isare B, Bouteiller L (2011) Chem Commun 47:10683–10685
Alshahateet SF, Bhadbhade MM, Bishop R, Scudderc ML (2015) CrystEngComm 17:877–888
Meot-Ner M, Elmore DE, Scheiner S (1999) J Am Chem Soc 121:7625–7635
Banerjee S, Adarsh NN, Dastidar P (2012) Cryst Growth Des 12:6061–6067
Guo H, Karplus M (2014) J Phys Chem 98:7104–7105
Du W, Yin QX, Gong JB, Bao Y, Zhang X, Sun XW, Ding SP, Xie C, Zhang MJ, Hao HX (2014) Cryst Growth Des 14:4519–4525
Kulkarni SA, McGarrity ES, Meekes H, Horst JH (2012) Chem Commun 48:4983–4985
Trask AV, Shan N, Motherwell WDS, Jones W, Feng SH, Tan RBH, Carpenter KJ (2015) Chem Commun 10:880–882
Choudhury AR, Nagarajan K, Row TNG (2006) CrystEngComm 8:482–488
Li P, Arman HD, Wang HL, Weng LH, Alfooty K, Angawi RF, Chen BL (2015) Cryst Growth Des 15:1871–1875
Yang WB, Greenaway A, Lin X, Matsuda R, Blake AJ, Wilson C, Lewis W, Hubberstey P, Kitagawa S, Champness NR, Schröder M (2010) J Am Chem Soc 132:14457–14469
Lü J, Perez-Krap C, Suyetin M, Alsmail NH, Yan Y, Yang SH, Lewis W, Bichoutskaia E, Tang CC, Blake AJ, Cao R, Schröder M (2014) J Am Chem Soc 136:12828–12831
Lemmerer A, Adsmond DA, Esterhuysen C, Bernstein J (2013) Cryst Growth Des 13:3935–3952
Wang L, Xue RY, Li YX, Zhao YR, Liu FQ, Huang KK (2014) CrystEngComm 16:7074–7089
Sheldrick GM (1997) SHELXS-97 Program for the solution of crystal structures. University of Gottingen, Gottingen
Sheldrick GM (1997) SHELXS-97 Programs for X-ray crystal structure refinement. University of Gottingen, Gottingen
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos. 51372125, 21571112, and 51572136), the Natural Science Foundation of Shandong Province, China (no. ZR2011BL015), and the State Key Laboratory of Inorganic Synthesis and Preparative Chemistry (2013-34).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
10870_2018_744_MOESM1_ESM.doc
CCDC reference numbers 1532370-1532371 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB 21EZ, UK; fax: (+44) 1223-336-033; or e-mail: deposit@ccdc.cam.ac.uk. Supplementary data associated with this article can be found in the online version. (DOC 492 KB)
Rights and permissions
About this article
Cite this article
Chen, C., Zhang, K., Zhang, C. et al. Solvent Control in the Formation of Supramolecular Solvates of 2,4-Diamino-6-methyl-1,3,5-triazine with 5-Nitroisophthalic Acid. J Chem Crystallogr 50, 1–7 (2020). https://doi.org/10.1007/s10870-018-0744-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-018-0744-0