Abstract
Manganese oxides, typically similar to δ-MnO2, form in the aquatic environment at near neutral pH via bacterially promoted oxidation of Mn(II) species by O2, as the reaction of [Mn(H2O)6]2+ with O2 alone is not thermodynamically favorable below pH of ~ 9. As manganese oxide species are reduced by the triphenylmethane compound leucoberbelein blue (LBB) to form the colored oxidized form of LBB (λmax = 623 nm), their concentration in the aquatic environment can be determined in aqueous environmental samples (e.g., across the oxic–anoxic interface of the Chesapeake Bay, the hemipelagic St. Lawrence Estuary and the Broadkill River estuary surrounded by salt marsh wetlands), and their reaction progress can be followed in kinetic studies. The LBB reaction with oxidized Mn solids can occur via a hydrogen atom transfer (HAT) reaction, which is a one-electron transfer process, but is unfavorable with oxidized Fe solids. HAT thermodynamics are also favorable for nitrite with LBB and MnO2 with ammonia (NH3). Reactions are unfavorable for NH4+ and sulfide with oxidized Fe and Mn solids, and NH3 with oxidized Fe solids. In laboratory studies and aquatic environments, the reduction of manganese oxides leads to the formation of Mn(III)-ligand complexes [Mn(III)L] at significant concentrations even when two-electron reductants react with MnO2. Key reductants are hydrogen sulfide, Fe(II) and organic ligands, including the siderophore desferioxamine-B. We present laboratory data on the reaction of colloidal MnO2 solutions (λmax ~ 370 nm) with these reductants. In marine waters, colloidal forms of Mn oxides (< 0.2 µm) have not been detected as Mn oxides are quantitatively trapped on 0.2-µm filters. Thus, the reactivity of Mn oxides with reductants depends on surface reactions and possible surface defects. In the case of MnO2, Mn(IV) is an inert cation in octahedral coordination; thus, an inner-sphere process is likely for electrons to go into the empty e *g conduction band of its orbitals. Using frontier molecular orbital theory and band theory, we discuss aspects of these surface reactions and possible surface defects that may promote MnO2 reduction using laboratory and field data for the reaction of MnO2 with hydrogen sulfide and other reductants.




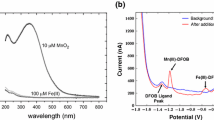
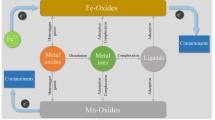
Reprinted with permission from Luther (2016)


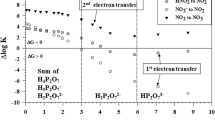
Reproduced and modified with permission from Luther (2016)



Similar content being viewed by others
References
Altmann HJ (1972) Determination of dissolved oxygen in water with leukoberbelin-blue I. A quick Winkler method. Anal Chem 262:97–99
Audi AA, Sherwood PMA (2002) Valence-band X-ray photoelectron spectroscopic studies of manganese and its oxides interpreted by cluster and band structure calculations. Surf Interface Anal 33:274–282
Bargar JR, Tebo BM, Bergmann U, Webb SM, Glatzel P, Chiu VQ, Villalobos M (2005) Biotic and abiotic products of Mn(II) oxidation by spores of the marine Bacillus sp. Strain SG-1. Am Mineral 90:143–154
Clement BG, Luther GW, Tebo BM (2009) Rapid, oxygen-dependent microbial Mn(II) oxidation kinetics at sub-micromolar oxygen concentrations in the Black Sea suboxic zone. Geochim Cosmochim Acta 73:1878–1889. https://doi.org/10.1016/j.gca.2008.12.023
Dénès F, Pichowicz M, Povie G, Renaud P (2014) Thiyl radicals in organic synthesis. Chem Rev 114:2587–2693
Duckworth OW, Sposito G (2005) Siderophore-manganese (III) interactions I. Manganite dissolution promoted by desferrioxamine-B. Environ Sci Technol 39:6045–6051
Duckworth OW, Sposito G (2007) Siderophore-promoted dissolution of synthetic and biogenic layer-type Mn oxides. Chem Geol 242:497–508
Estes ER, Andeer PF, Nordlund D, Wankel SD, Hansel CM (2016) Biogenic manganese oxides as reservoirs of organic carbon and proteins in terrestrial and marine environments. Geobiology 15:158–172. https://doi.org/10.1111/gbi.12195
Foti MC, Johnson ER, Vinqvist MR, Wright JS, Barclay LRC, Ingold KU (2002) Naphthalene diols: a new class of antioxidants intramolecular hydrogen bonding in catechols, naphthalene diols, and their aryloxyl radicals. J Org Chem 67:5190–5196
Gardner KA, Kuehnert LL, Mayer JM (1997) Hydrogen atom abstraction by permanganate: oxidations of arylalkanes in organic solvents. Inorg Chem 36:2069–2078
Herszage J, dos Santos Afonso M (2003) Mechanism of hydrogen sulfide oxidation by manganese(IV) oxide in aqueous solutions. Langmuir 19:9684–9692
Javanaud C, Michotey V, Guasco S, Garcia N, Anschutz P, Canton M, Bonin P (2011) Anaerobic ammonium oxidation mediated by Mn-oxides: from sediment to strain level. Res Microbiol 162:848–857
Kartal B, de Almeida NM, Maalcke WJ, Op den Camp HJM, Jetten MSM, Keltjens JT (2013) How to make a living from anaerobic ammonium oxidation. FEMS Microbiol Rev 37:428–461
Konovalov S, Luther GW, Friederich GE, Nuzzio DB, Tebo BM, Murray JW, Oguz T, Glazer B, Trouwborst RE, Clement B, Murray KJ, Romanov AS (2003) Lateral injection of oxygen with the Bosporus plume: fingers of oxidizing potential in the Black Sea. Limnol Oceanogr 48:2369–2376
Learman DL, Voelker BM, Vazquez-Rodriguez AI, Hansel CM (2011a) Formation of manganese oxides by bacterially generated superoxide. Nat Geosci 4:95–98
Learman DL, Wankel SD, Webb SM, Martinez N, Madden AS, Hansel CM (2011b) Coupled biotic–abiotic Mn(II) oxidation pathway mediates the formation and structural evolution of biogenic Mn oxides. Geochim Cosmochim Acta 75:6048–6063. https://doi.org/10.1016/j.gca.2011.07.026
Lin H, Taillefert M (2014) Key geochemical factors regulating Mn(IV)-catalyzed anaerobic nitrification in coastal marine sediments. Geochim Cosmochim Acta 133:17–33
Lucarini M, Pedrielli P, Pedulli GF (1996) Bond dissociation energies of O–H bonds in substituted phenols from equilibration studies. J Org Chem 61:9259–9263
Lucarini M, Mugnaini V, Pedulli GF (2002) Bond dissociation enthalpies of polyphenols: the importance of cooperative effects. J Org Chem 67:928–931
Luther GW (2010) The role of one and two electron transfer reactions in forming thermodynamically unstable intermediates as barriers in multi-electron redox reactions. Aquat Geochem 16:395–420
Luther GW (2016) Inorganic chemistry for geochemistry and environmental sciences: fundamentals and applications. Wiley, New York
Luther GW, Popp JI (2002) Kinetics of the abiotic reduction of polymeric manganese dioxide by nitrite: an anaerobic nitrification reaction. Aquat Geochem 8:15–36
Luther GW, Sundby B, Lewis BL, Brendel PJ, Silverberg N (1997) Interactions of manganese with the nitrogen cycle: alternative pathways to dinitrogen. Geochim Cosmochim Acta 61:4043–4052
Madison AS, Tebo BM, Mucci A, Sundby B, Luther GW (2013) Abundant Mn(III) in porewaters is a major component of the sedimentary redox system. Science 341:875–878. https://doi.org/10.1126/science.1241396
Matocha CJ, Sparks DL, Amonette JE, Kukkadapu RK (2001) Kinetics and mechanism of birnessite reduction by catechol. Soil Sci Soc Am J 65:58–66
Mayer JM (2011) Understanding hydrogen atom transfer: from bond strengths to marcus theory. Acc Chem Res 44:36–46
Morse JW, Luther GW (1999) Chemical influences on trace metal-sulfide interactions in anoxic sediments. Geochim Cosmochim Acta 63:3373–3378
Oldham VE, Owings SM, Jones M, Tebo BM, Luther GW (2015) Evidence for the presence of strong Mn(III)-binding ligands in the water column of the Chesapeake Bay. Mar Chem 171:58–66. https://doi.org/10.1016/j.marchem.2015.02.008
Oldham VE, Mucci A, Tebo BM, Luther GW (2017a) Soluble Mn(III)-L complexes are ubiquitous in oxygenated waters and stabilized by humic ligands. Geochim Cosmochim Acta 199:238–246. https://doi.org/10.1016/j.gca.2016.11.043
Oldham VE, Jones MR, Tebo BM, Luther GW (2017b) Oxidative and reductive processes contributing to manganese cycling at oxic–anoxic interfaces. Mar Chem 195:122–128. https://doi.org/10.1016/j.marchem.2017.06.002
Oldham VE, Miller MT, Jensen LT, Luther GW (2017c) Revisiting Mn and Fe removal in humic rich estuaries. Geochim Cosmochim Acta 209:267–283. https://doi.org/10.1016/j.gca.2017.04.001
Perez-Benito JF, Brillas E, Pouplana R (1989) Identification of a soluble form of colloidal manganese (IV). Inorg Chem 28:390–392
Rickard D, Luther GW (2007) Chemistry of iron sulfides. Chem Rev 107:514–562
Salter-Blanc AJ, Bylaska EJ, Lyon MA, Ness SC, Tratnyek PG (2016) Structure–activity relationships for rates of aromatic amine oxidation by manganese dioxide. Environ Sci Technol 50:5094–5102
Sheriff TS, Carr P, Piggott B (2003) Manganese catalysed reduction of dioxygen to hydrogen peroxide: structural studies on a manganese(III)-catecholate complex. Inorg Chim Acta 348:115–122
Siebecker MG, Madison AS, Luther GW (2015) Reduction kinetics of polymeric (soluble) manganese (IV) oxide (MnO2) by ferrous iron (Fe2+). Aquat Geochem 21:143–158. https://doi.org/10.1007/s10498-015-9257-z
Stone AT, Morgan JJ (1984a) Reduction and dissolution of manganese (III) and manganese(IV) oxides by organics. 1. Reaction with hydroquinone. Environ Sci Technol 18:450–456
Stone AT, Morgan JJ (1984b) Reduction and dissolution of manganese (III) and manganese(IV) oxides by organics. 2. Survey of the reactivity of organics. Environ Sci Technol 18:617–624
Taube H (1983) Electron transfer between metal complexes—retrospective. Nobel Lecture, 8 December 1983
Tebo BM, Bargar JR, Clement B, Dick G, Murray KJ, Parker D, Verity R, Webb SM (2004) Biogenic manganese oxides: properties and mechanisms of formation. Annu Rev Earth Planet Sci 32:287–328
Wright MH, Geszvain K, Oldham VE, Luther GW, Tebo BM (2018) Oxidative formation and removal of complexed Mn(III) by pseudomonas. Front Microbiol 9:560. https://doi.org/10.3389/fmicb.2018.00560
Yao W, Millero FJ (1993) The rate of sulfide oxidation by δMnO2 in seawater. Geochim Cosmochim Acta 57:3359–3365
Yao W, Millero FJ (1995) Oxidation of hydrogen sulfide by Mn(IV) and Fe(III) (hydr)oxides in seawater. In: Vairavamurthy V, Schoonen MAA, Eglinton TI, Luther GW, Manowitz B (eds) Geochemical transformations of sedimentary sulfur. American chemical society symposium series, vol 612, Ch. 14. Washington, DC, pp 260–279
Zhang X-M, Bordwell FG (1992) Homolytic bond dissociation energies of the benzylic C–H bonds in radical anions and radical cations derived from fluorenes, triphenylmethanes, and related compounds. J Am Chem Soc 114:9787–9792
Acknowledgements
This work was funded by grants from the Chemical Oceanography program of the National Science Foundation (OCE-1558738 to GWL; OCE-1558692 to BMT). We thank A. Mucci for constructive comments on an earlier version of this manuscript. We also thank the anonymous reviewers for their constructive comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luther, G.W., Thibault de Chanvalon, A., Oldham, V.E. et al. Reduction of Manganese Oxides: Thermodynamic, Kinetic and Mechanistic Considerations for One- Versus Two-Electron Transfer Steps. Aquat Geochem 24, 257–277 (2018). https://doi.org/10.1007/s10498-018-9342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-018-9342-1