Abstract
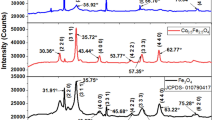
Bis-(N-methylbenzyldithiocarbamato)iron(II) complex was thermolyzed at 120, 180 and 240 °C to investigate the effects of temperature on the structural and optical properties of the as-prepared iron sulfide nanocrystals. Powder X-ray diffraction studies revealed a pyrrhotite-4M (Fe7S8) crystalline phase for iron sulfide nanocrystals (FeS1) obtained at 120 °C and pyrrhotite-6C (Fe11S12) phases for iron sulfide nanocrystals (FeS2 and FeS3) obtained at 180 and 240 °C. HRTEM images confirmed the crystallite sizes of the iron sulfide nanoparticles are in the range 2.04–4.77 for FeS1 obtained at 120 °C, 4.82–11.12 for FeS2 obtained at 180 °C and 6.50–12.39 nm for FeS3 iron sulfide nanocrystals obtained at 240 °C. These results confirmed that increase in temperature resulted in the formation of iron sulfide nanocrystals with larger diameter. The optical band gaps (Eg) of the iron sulfide nanocrystals are in the range 3.70–3.76 eV. The iron sulfide nanocrystals were incorporated into hydroxyethyl cellulose (HEC) matrix to prepare iron sulfide/HEC nanocomposites.










Similar content being viewed by others
References
Y. Wu, D. Wang, Y. Li, Nanocrystals from solutions: catalysts. Chem. Soc. Rev. 43, 2112–2124 (2014)
J.M. Costa-Fernández, R. Pereiro, A. Sanz-Medel, The use of luminescent quantum dots for optical sensing. Trends Anal. Chem. 25, 207–218 (2006)
Z.H. Farooqi, S.R. Khan, R. Begum, A. Ijaz, Review on synthesis, properties, characterization, and applications of responsive microgels fabricated with gold nanostructures. Rev. Chem. Eng. 32, 49–69 (2016)
Y. Lu, Y. Chen, R.A. Gemeinhart, W. Wu, T. Li, Developing nanocrystals for cancer treatment. Nanomedicine 10, 2537–2552 (2015)
K.M.M. Abou El-Nour, A.A. Eftaiha, A. Al-Warthan, R.A.A. Ammar, Synthesis and applications of silver nanoparticles. Arab. J. Chem 3, 135–140 (2010)
X. Shi, K. Sun, L.P. Balogh, J.R. Baker, Synthesis, characterization, and manipulation of dendrimer-stabilized iron sulfide nanoparticles. Nanotechnology 17, 4554–4560 (2006)
P.D. Matthews, M. Akhtar, M.A. Malik, N. Revaprasadu, P. O’Brien, Synthetic routes to iron chalcogenide nanoparticles and thin films. Dalton Trans. 45, 18803–18812 (2016)
L. Fei, Y. Jiang, Y. Xu, G. Chen, Y. Li, X. Xu, S. Deng, H. Luo, A novel solvent-free thermal reaction of ferrocene and sulfur for one-step synthesis of iron sulfide and carbon nanocomposites and their electrochemical performance. J. Power Sources 265, 1–5 (2014)
S. Mlowe, D.J. Lewis, M.A. Malik, J. Raftery, E.B. Mubofu, P. O’Brien, N. Revaprasadu, Heterocyclic dithiocarbamato-iron (III) complexes: single-source precursors for aerosol-assisted chemical vapour deposition (AACVD) of iron sulfide thin films. Dalton Trans. 45, 2647–2655 (2016)
M. Akhtar, M.A. Malik, F. Tuna, P. O’Brien, The synthesis of iron sulfide nanocrystals from tris (O-alkylxanthato) iron (III) complexes. J. Mater. Chem. 1, 8766–8774 (2013)
M. Akhtar, J. Akhter, M.A. Malik, P. O’Brien, F. Tuna, J. Raftery, M. Helliwell, Deposition of iron sulfide nanocrystals from single source precursors. J. Mater. Chem. 21, 9737–9745 (2011)
W. Han, M. Gao, Investigations on iron sulfide nanosheets prepared via a single-source precursor approach. Cryst. Growth Des. 8, 1023–1030 (2008)
S. Mlowe, S.S. Garje, T. Moyo, N. Revaprasadu, Magnetic iron sulfide nanoparticles for potential applications in gas sensing. MRS Adv. 1, 235–240 (2016)
A. Akhoondi, M. Aghaziarati, N. Khandan, Production of highly pure iron disulfide nanoparticles using hydrothermal synthesis method. Appl. Nanosci. 3, 417–422 (2013)
J.T. Lue, Physical properties of nanomaterials. Encyclopedia of Nanoscience and Nanotechnology, Edited by H.S. Nalwa, 2007, X, 1–46
P.A. Ajibade, A.M. Paca, Tris(dithiocarbamato)iron(III) complexes as precursors for iron sulfide nanocrystals and iron sulfide-hydroxyethyl cellulose composites. J. Sulfur. Chem. 40(1), 52–64 (2019)
C.J. Barrelet, Y. Wu, D.C. Bell, C.M. Lieber, Synthesis of CdS and ZnS nanowires using single-source molecular precursors. J. Am. Chem. Soc. 125, 11498–11499 (2003)
M.A. Malik, N. Revaprasadu, P. O’Brien, Air-stable single-source precursors for the synthesis of chalcogenide semiconductor nanoparticles. Chem. Mater. 13, 913–920 (2001)
T. Trindade, P. O’Brien, X.-M. Zhang, Synthesis of CdS and CdSe nanocrystallites using a novel single-molecule precursors approach. Chem. Mater. 9, 523–530 (1997)
P.A. Ajibade, B.C. Ejelonu, Group 12 dithiocarbamate complexes: synthesis, spectral studies and their use as precursors for metal sulfides nanoparticles and nanocomposites. Spectrochim. Acta A 113, 408–414 (2013)
A.M. Paca, P.A. Ajibade, Synthesis and structural studies of iron sulphide nanocomposites prepared from Fe(III) dithiocarbamates single source precursors. Mater. Chem. Phys. 202, 143–150 (2017)
C. Buchmaier, M. Glänzer, A. Torvisco, P. Poelt, K. Wewerka, B. Kunert, K. Gatterer, G. Trimmel, T. Rath, Nickel sulfide thin films and nanocrystals synthesized from nickel xanthate precursors. J. Mater. Sci. 52, 10898–10914 (2017)
K. Cavell, J. Hill, R. Magee, Synthesis and characterisation of some secondary amine-dithiocarbamate salts. J. Inorg. Nucl. Chem. 41, 1277–1280 (1979)
P. Vanitha, P. O’Brien, Phase control in the synthesis of magnetic iron sulfide nanocrystals from a cubane-type Fe–S cluster. J. Am. Chem. Soc. 130, 17256–17257 (2008)
T. Duan, W. Lou, X. Wang, Q. Xue, Size-controlled synthesis of orderly organized cube-shaped lead sulfide nanocrystals via a solvothermal single-source precursor method. Colloids Surf. A 310, 86–93 (2007)
S. Sibokoza, M. Moloto, N. Moloto, P. Sibiya, The effect of temperature and precursor concentration on the synthesis of cobalt sulphide nanoparticles using cobalt diethyldithiocarbamate complex. Chalcogenide Lett. 14, 69–78 (2017)
S. Santhi, C. Radhakrishnan Namboori, Synthesis, characterization and spectral studies of Fe(III) and Cr(III) schiff base complexes with acetoacetanilidoethylenediamine. Orient. J. Chem. 27, 1203–1208 (2011)
S.M. Mamba, A.K. Mishra, B.B. Mamba, P.B. Njobeh, M.F. Dutton, E. Fosso-Kankeu, Spectral, thermal andin vitroantimicrobial studies of cyclohexylamine-N-dithiocarbamate transition metal complexes. Spectrochim. Acta A 77, 579–587 (2010)
F.P. Andrew, P.A. Ajibade, Synthesis, characterization, and electrochemical studies of Co(II, III) dithiocarbamate complexes. J. Coord. Chem. 72(5–7), 1171–1186 (2019)
G. Kharadi, Effect of the thermal decomposition and in vitro antimicrobial activity of mixed ligand copper(II) complexes. Synth. React. Inorg. Met-Organ. Nano-Met. Chem. 42, 424–431 (2012)
E.-S.A. El-Samanody, A.K. El-Sawaf, M. Madkour, Synthesis, crystal structure, spectral and thermal investigations of morpholinyldithiocarbamate complexes: a novel coordinated precursors for efficient metal oxide nanophotocatalysts. Inorg. Chim. Acta 487, 307–315 (2019)
V.D. Benedini, P.A. Antunes, É.T. Cavalheiro, G.O. Chierice, Thermoanalytical and solution stability studies of hexamethylenedithiocarbamates. J. Braz. Chem. Soc. 17, 680–688 (2006)
P.A. Ajibade, J.Z. Mbese, B. Omondi, Group 12 dithiocarbamate complexes: synthesis, characterization and X-ray crystal structures of Zn(II) and Hg(II) complexes and their use as precursors for metal sulfide nanoparticles. Nano- Met Chem. 47(2), 202–212 (2017)
J. Watson, B. Cressey, A. Roberts, D. Ellwood, J. Charnock, A. Soper, Structural and magnetic studies on heavy-metal-adsorbing iron sulphide nanoparticles produced by sulphate-reducing bacteria. J. Magn. Magn. Mater. 214, 13–30 (2000)
J.Z. Mbese, P.A. Ajibade, Synthesis, spectroscopic, structural and optical studies of Ru2S3 nanoparticles prepared from single-source molecular precursors. J. Mol. Struct. 1143, 274–281 (2017)
N.L. Botha, P.A. Ajibade, Effect of temperature on crystallite sizes of copper sulfide nanocrystals prepared from copper(II) dithiocarbamate single source precursor. Mater. Sci. Semicond. 43, 149–154 (2016)
W. Ming-Dong, Z. Dao-Yun, L. Yi, Z. Lin, Z. Chang-Xi, H. Zhen-Hui, C. Di-Hu, W. Li-Shi, Determination of thickness and optical constants of ZnO thin films prepared by filtered cathode vacuum arc deposition. Chin. Phys. Lett. 25, 743–746 (2008)
J.Z. Mbese, P.A. Ajibade, A simple route to ruthenium sulfide nanoparticles via thermal decomposition of precursor complexes. J. Nano Res. 54, 158–171 (2018)
Acknowledgements
The authors gratefully appreciate the financial support from National Research Foundation and Sasol, South Africa.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ajibade, P.A., Paca, A.M. The Effects of Temperature on Iron Sulfide Nanocrystals Prepared from Thermal Decomposition of Bis-(N-methylbenzyldithiocarbamato)iron(II) Complex. J Inorg Organomet Polym 30, 1327–1338 (2020). https://doi.org/10.1007/s10904-019-01264-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10904-019-01264-3