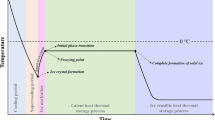
Abstract
Freezing is a widely used method to extend the shelf life of foods. However, the frozen products are subjected to temperature fluctuations during storage and shipping, which cause events such as ice recrystallization and moisture/mass migration that further damage food quality. This paper reviews the mechanisms of ice crystal formation, propagation, and glass transition and presents experimental results about the effect of freeze-thaw cycles on ice recrystallization. The mathematical models for prediction of freezing time and description of heat and mass transfer during the freezing process are also addressed. The crystal formation and moisture/mass mobility involve multiscale characteristics, while the traditional modeling can only describe single-scale averaged effects. Therefore, the hybrid mixture theory (HMT), which is based on multiscale balance laws and entropy inequality, is also discussed in the context of the freezing problem to predict phase change, crystal growth, and thermomechanical effects.







Similar content being viewed by others
Abbreviations
- a :
-
Thickness/diameter (m)
- B α :
-
Mixture viscosity of the biopolymeric matrix (Pa s)
- B if :
-
Biot number
- c app :
-
Apparent specific heat (J/(kg K))
- c p :
-
Specific heat (J/ (kg K))
- D w :
-
Coefficient of diffusivity (m2/s)
- \( {}^{\beta}{\widehat{e}}^{\alpha} \) :
-
Net rate of mass transfer from phase β to phase α (kg/(m3 s))
- E f :
-
Shape factor
- h :
-
Heat transfer coefficient (W/ (m2 k))
- H :
-
Specific enthalpy (J/Kg3)
- H w :
-
Enthalpy of the diffusing moisture/solute (J/Kg3)
- k :
-
Thermal conductivity (W/ (m K))
- K α :
-
Permeability of the α phase (m2)
- L f :
-
Latent heat of freezing (J/Kg)
- \( {\dot{m}}_w \) :
-
Mass flux (kg/(m2 s))
- m, n, A, B:
- p α :
-
Physical pressure in phase α (Pa)
- P, Q:
-
Shape factors in Eq. (3)
- t :
-
Time (s)
- t f :
-
Freezing time (s)
- t t :
-
Thawing time (s)
- t plank :
-
Freezing time in the Plank equation(s)
- T :
-
Temperature (K)
- T a /T b :
-
Cooling/heating medium temperature (K)
- T c :
-
Thermal center temperature (K)
- T cr :
-
Initial freezing temperature (K)
- T f :
-
Initial freezing temperature (K)
- \( {\boldsymbol{v}}_l^{\alpha, s} \) :
-
Relative velocity of α to β phase (m/s)
- W :
-
Concentration of the compound
- ε α :
-
Volume fraction of α phase
- μ α :
-
Shear viscosity of α phase (Pa s)
- ρ α :
-
Density of phase α (kg/m3)
- ƛ:
-
Latent heat (J/Kg)
- α :
-
General representation of a phase
- β :
-
General representation of a phase
- s :
-
Solid phase
- w :
-
Water phase
- g :
-
Gas phase
- D α/Dt :
-
Material time derivative with respect to velocity of α phase particle (s−1)
- Dot(⋅)D s/Dt :
-
Material time derivative with respect to velocity of solid phase particle
- ∇:
-
Del operator
References
Sharma A (2014) Global frozen food market trends and forecasts To 2020. Retrieve from https://www.linkedin.com/pulse/20141118100129-214337948-global-frozen-food-market-trends-and-forecasts-to-2020.
Sun D-W (2011) Handbook of frozen food processing and packaging. CRC Press, Boca Raton
Adapa S, Schmidt K, Jeon I, Herald T, Flores R (2000) Mechanisms of ice crystallization and recrystallization in ice cream: a review. Food Rev Int 16(3):259–271
McDonald K, Sun D-W (2000) Vacuum cooling technology for the food processing industry: a review. J Food Eng 45(2):55–65
Flores A, Goff H (1999) Ice crystal size distributions in dynamically frozen model solutions and ice cream as affected by stabilizers. J Dairy Sci 82(7):1399–1407
Szymońska J, Wodnicka K (2005) Effect of multiple freezing and thawing on the surface and functional properties of granular potato starch. Food Hydrocoll 19(4):753–760
Ullah J, Takhar PS, Sablani SS (2014) Effect of temperature fluctuations on ice-crystal growth in frozen potatoes during storage. LWT-Food Sci Technol 59(2):1186–1190
Goff H, Sahagian M (1996) Glass transitions in aqueous carbohydrate solutions and their relevance to frozen food stability. Thermochim Acta 280:449–464
Delgado A, Sun D-W (2001) Heat and mass transfer models for predicting freezing processes–a review. J Food Eng 47(3):157–174
Pham QT (2006) Modelling heat and mass transfer in frozen foods: a review. Int J Refrig 29(6):876–888
Patel S, Venditti RA, Pawlak JJ, Ayoub A, Rizvi SS (2009) Development of cross-linked starch microcellular foam by solvent exchange and reactive supercritical fluid extrusion. J Appl Polym Sci 111(6):2917–2929
Singh PP, Cushman JH, Maier DE (2003) Three scale thermomechanical theory for swelling biopolymeric systems. Chem Eng Sci 58(17):4017–4035
Takhar PS (2014) Unsaturated fluid transport in swelling poroviscoelastic biopolymers. Chem Eng Sci 109:98–110
Reid DS (1998) Freezing-nucleation in foods and antifreeze actions, the properties of water in foods. Springer, New York, pp 275–286
Slade L, Levine H (1991) A food polymer science approach to structure-property relationships in aqueous food systems: non-equilibrium behavior of carbohydrate-water systems, water relationships in foods. Springer, New York, pp 29–101
Goff HD (1992) Low-temperature stability and the glassy state in frozen foods. Food Res Int 25(4):317–325
Reid DS (1997) Overview of physical/chemical aspects of freezing, quality in frozen food. Springer, New York, pp 10–28
Franks F (1993) Solid aqueous solutions. Pure Appl Chem 65(12):2527–2537
Abiad M, Carvajal M, Campanella O (2009) A review on methods and theories to describe the glass transition phenomenon: applications in food and pharmaceutical products. Food Eng Rev 1(2):105–132
Fox TG Jr, Flory PJ (1950) Second-order transition temperatures and related properties of polystyrene. I. Influence of molecular weight. J Appl Phys 21(6):581–591
Ward IM, Sweeney J (2004) Experimental studies of linear viscoelastic behaviour as a function of frequency and temperature: time– temperature equivalence. Mechanical properties of solid polymers. John Wiley and Sons, Chichester, pp 135–165
Roos Y (1992) Phase transitions and transformations in food systems. Handbook of food engineering. pp 145–197
Gibbs JH, DiMarzio EA (1958) Nature of the glass transition and the glassy state. J Chem Phys 28(3):373–383
Roos YH (2010) Glass transition temperature and its relevance in food processing. Annu Rev Food Sci Technol 1:469–496
Roos YH, Drusch S (2015) Phase transitions in foods. Academic Press, Cambridge
Roos Y, Karel M (1991) Amorphous state and delayed ice formation in sucrose solutions. Int J Food Sci Technol 26(6):553–566
Levine H, Slade L (1990) Cryostabilization technology: thermoanalytical evaluation of food ingredients and systems. Elsevier Applied Science, London
Noel TR, Ring SG, Whittam MA (1990) Glass transitions in low-moisture foods. Trends Food Sci Technol 1:62–67
Luyet B, Rasmussen D (1967) Study by differential thermal analysis of the temperatures of instability of rapidly cooled solutions of glycerol, ethylene glycol, sucrose and glucose. Biodynamica 10(210):167–191
Buera MP, Roos Y, Levine H, Slade L, Corti HR, Reid DS, Auffret T, Angell CA (2011) State diagrams for improving processing and storage of foods, biological materials, and pharmaceuticals (IUPAC technical report). Pure Appl Chem 83(8):1567–1617
Winger R (1984) Storage life and eating related quality of new-Zealand frozen lamb: a compendium of irrepressible longevity. Thermal processing and quality of foods. Elsevier, London
Alvarez MD, Canet W (1998) Effect of temperature fluctuations during frozen storage on the quality of potato tissue (cv. Monalisa). Zeitschrift für Lebensmitteluntersuchung und-Forschung A 206(1):52–57
Bustabad OM (1999) Weight loss during freezing and the storage of frozen meat. J Food Eng 41(1):1–11
Pham Q, Mawson R (1997) Moisture migration and ice recrystallization in frozen foods, quality in frozen food. Springer, New York, pp 67–91
Cutting C, Malton R (1974) Evaporative losses in the commercial freezing and storage of meat. Meat Res Inst Symp 36:31–37
Pham Q, Durbin J, Willix J (1982) Survey of weight loss from lamb in frozen storage. Int J Refrig 5(6):337–342
Syamaladevi RM, Manahiloh KN, Muhunthan B, Sablani SS (2012) Understanding the influence of state/phase transitions on ice recrystallization in Atlantic salmon (Salmo salar) during frozen storage. Food Biophysics 7(1):57–71
Do G, Araki T, Bae Y, Ishikura K, & Sagara Y (2015) Three-dimensional measurement of ice crystals in frozen materials by near-infrared imaging spectroscopy. Drying Techno 33(13) 1614–1620
Mousavi R, Miri T, Cox PW, Fryer PJ (2005) A novel technique for ice crystal visualization in frozen solids using X [ray micro] computed tomography. J Food Sci 70(7):e437–e442
Ablett S, Clarke CJ, Izzard MJ, Martin DR (2002) Relationship between ice recrystallisation rates and the glass transition in frozen sugar solutions. J Sci Food Agric 82(15):1855–1859
Goff H (1997) Measurement and interpretation of the glass transition in frozen foods, quality in frozen food. Springer, New York, pp 29–50
Regand A, Goff H (2002) Effect of biopolymers on structure and ice recrystallization in dynamically frozen ice cream model systems. J Dairy Sci 85(11):2722–2732
Miller-Livney T, Hartel RW (1997) Ice recrystallization in ice cream: interactions between sweeteners and stabilizers. J Dairy Sci 80(3):447–456
Sutton RL, Wilcox J (1998) Recrystallization in ice cream as affected by stabilizers. J Food Sci 63(1):104–107
James S, James C, Evans J (2006) Modelling of food transportation systems—a review. Int J Refrig 29(6):947–957
Heldman D, Taylor T (1997) Modeling of food freezing, quality in frozen food. Springer, New York, pp 51–64
Pham QT (2014) Food freezing and thawing calculations. Springer, New York
Fellows PJ (2009) Food processing technology: principles and practice. Elsevier, London
Pham Q (1986) Simplified equation for predicting the freezing time of foodstuffs. Int J Food Sci Technol 21(2):209–219
Cleland AC (1990) Food refrigeration processes: analysis, design and simulation. Elsevier Applied Science, London
Salvadori V, Mascheroni R (1991) Prediction of freezing and thawing times of foods by means of a simplified analytical method. J Food Eng 13(1):67–78
Salvadori VO, Reynoso RO, De Michelis A, Mascheroni RH (1987) Freezing time predictions for regular shaped foods: a simplified graphical method. Int J Refrig 10(6):357–361
Heldman D (1982) Food properties during freezing. Food Technol 36(2):92–96
Cleland DJ, Cleland AC, Earle RL, Byrne SJ (1987) Prediction of freezing and thawing times for multi-dimensional shapes by numerical methods. Int J Refrig 10(1):32–39
Ozisik N (1994) Finite difference methods in heat transfer. CRC press, Boca Raton
Ansari F (1999) Finite difference solution of heat and mass transfer problems related to precooling of food. Energy Convers Manag 40(8):795–802
Sheen S, Hayakawa K (1990) Finite difference analysis for the freezing or thawing of an irregular food with volumetric change. Eng Food 2:426–441
Campañone LA, Salvadori VO, Mascheroni RH (2005) Food freezing with simultaneous surface dehydration: approximate prediction of weight loss during freezing and storage. Int J Heat Mass Transf 48(6):1195–1204
Cushman JH (1997) The physics of fluids in hierarchical porous media: angstroms to miles. Springer Science & Business Media, New York
Coleman BD, Noll W (1963) The thermodynamics of elastic materials with heat conduction and viscosity. Arch Ration Mech Anal 13(1):167–178
Hassanizadeh M, Gray WG (1979) General conservation equations for multi-phase systems: 1. Averaging procedure. Adv Water Resour 2:131–144
Ditudompo S, Takhar PS (2015) Hybrid mixture theory based modeling of transport mechanisms and expansion-thermomechanics of starch during extrusion. AICHE J 61(12):4517–4532
Takhar PS, Maier DE, Campanella OH, Chen G (2011) Hybrid mixture theory based moisture transport and stress development in corn kernels during drying: validation and simulation results. J Food Eng 106(4):275–282
Acknowledgements
Thanks to USDA-NIFA for providing financial support under Award No. 2015-67017-23074.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author has previously published as Pawan P. Singh
Rights and permissions
About this article
Cite this article
Zhao, Y., Takhar, P.S. Freezing of Foods: Mathematical and Experimental Aspects. Food Eng Rev 9, 1–12 (2017). https://doi.org/10.1007/s12393-016-9157-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12393-016-9157-z