Abstract
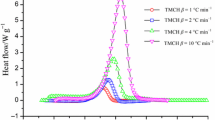
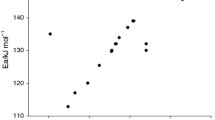
Severe fire and explosions are frequent phenomena during handling of organic peroxides that are promoted supremely by conditions such as chemical impurities and thermal instability. As an initiator in the polymerization process, cumene hydroperoxide (CHP) has wide usage in the chemical process industry. This violently reactive chemical is studied here experimentally using differential scanning calorimeter (DSC), an isothermal mode of operation that can access the thermal hazards in the decomposition of CHP alone and later mixed with products following an autocatalytic reaction scheme. Importantly, DSC-evaluated thermokinetic parameters such as reaction enthalpy (ΔHd), time to maximum rate (TMRiso), and maximum heat flow (Qmax) were estimated to ascertain the degree of thermal hazard under various transportation and storage temperatures. The Heat-Wait-Search mode of accelerating rate calorimeter has been used to investigate decomposition kinetics parameters data under an adiabatic condition. Data such as initial exothermic temperature (T0), self-heating rate (dT/dt), pressure rise rate (dP/dt) and pressure–temperature profiles help to gauge the runaway reaction hazard of CHP alone and then mixed with its products to support the autocatalytic model of exothermic decomposition. The curve fitting data indicated that activation energy had reduced from 245.4 to 236.7 and 242.3 kJ mol−1, when CHP was mixed with acetone or dicumyl peroxide, respectively. The decrease in activation energy for autocatalytic material thermal decomposition reaction is depicted here with various experimental findings and mathematical analysis.









Similar content being viewed by others
Abbreviations
- A :
-
Pre-exponential factor (s−1)
- c vb :
-
Average heat capacity of the bomb (J g−1 K−1)
- c vs :
-
Average heat capacity of the mass (J g−1 K−1)
- dP/dt :
-
Pressure rise rate (bar min−1)
- dT/dt :
-
Self-heating rate (°C min−1)
- E a :
-
Apparent activation energy (kJ mol−1)
- K :
-
Reaction rate constant
- M b :
-
Mass of bomb (mg)
- M s :
-
Mass of sample (mg)
- N :
-
Reaction order
- Q peak :
-
Peak power of the reaction (W g−1)
- R:
-
Molar gas constant (8.314 J mol−1 K−1)
- r T :
-
Self-heating rate (°C min−1)
- T :
-
Reaction temperature (°C)
- TMRiso :
-
Time to maximum rate under isothermal (min)
- T f,exo :
-
Final temperature of the exothermic reaction (°C)
- ∆Tad :
-
Adiabatic temperature rise (°C)
- ∆Tab :
-
Absolute temperature rise (°C)
- β :
-
Heating rates (°C min−1)
- Φ :
-
Thermal inertia (dimensionless)
References
Hou HY, Shu CM, Tsai TL. Reactions of cumene hydroperoxide mixed with sodium hydroxide. J Hazard Mater. 2008;152:1214–9.
Huang D, Han M, Wang J, Jin Y. Catalytic decomposition process of cumene hydroperoxide using sulfonic resins as catalyst. ChemEng J. 2002;88:215–23.
Lu KT, Luo KM, Lin SH, Su SH, Hu KH. The acid-catalyzed phenol-formaldehyde reaction critical runaway conditions and stability criterion. Process Saf Environ Protect. 2004;82:37–47.
Schmidt RJ. Industrial catalytic processes-phenol production. Appl Catal A Gen. 2005;208:89–108.
Sato H, Shimizu T. Marked effects of alcohols and imidazoles on the cumyl hydroperoxide reaction with the wild-type cytochrome P450 1A21. Arch Biochem Biophys. 1995;322:277–83.
Shen SJ, Wu SH, Chi CC, Horng JJ, Shu CM. Simulation of solid thermal explosion and liquid thermal explosion of dicumyl peroxide using calorimetric technique. Simul Model Pract Theory. 2011;19:1251–7.
Hou HY, Liao TS, Duh YS, Shu CM. Reactive incompatibility of cumene hydroperoxide mixed with alkaline solutions. J Therm Anal Calorim. 2006;85(1):145–50.
Huang D, Han M, Wang J. Catalytic decomposition process of cumene hydroperoxide using sulfonic resins as catalyst. ChemEng J. 2002;88:215–23.
Luttrell BW. Toxic tips: Phenol. Chem Health Saf. 2003;3:1074–9098.
Hsu JM, Su MS, Huang CY, Duh YS. Calorimetric studies and lessons on fires and explosions of a chemical plant producing CHP and DCPO. J Hazard Mater. 2012;17–18:19–28.
Iwata Y, Momota M, Koseki H. Thermal risk evaluation of organic peroxide by automatic pressure tracking adiabatic calorimeter. J Therm Anal Calorim. 2006;85:617–22.
Luo KM, Chang JG, Lin SH, Chang CT, Yeh TF, Hu KH, Kao CS. The criterion of critical runaway and stable temperatures in cumene hydroperoxide reaction. J Loss Prev Process Ind. 2001;14:229–39.
Zhu QC, Shen BX, Ling H, Gu R. Cumene hydrogenation over Pd/C catalysts. J Hazard Mater. 2010;175:646–50.
Ho TC, Duh YS, Chen JR. Case studies of incidents in runaway reactions and emergency relief. Process Saf Progress. 2004;17(4):259–62.
Kletz TA. Fires and explosions of hydrocarbon oxidation plants. Plant/Oper Progress. 1988;7:226–30.
Levin ME, Gonzales NO, Zimmerman LW, Yang J. Kinetics of acid-catalyzed the cleavage of cumene hydroperoxide. J Hazard Mater. 2006;130:88–106.
Hou HY, Su CH, Shu CM. Thermal risk analysis of cumene hydroperoxide in the presence of alkaline catalysts. J Loss Prev Process Ind. 2012;25:176–80.
Talouba IB, Balland L, Mouhab N, Abdelghani-Idrissi MA. Kinetics parameter estimation for decomposition of organic peroxides by means of DSC measurements. J Loss Prev Process Ind. 2011;24:391–6.
Cao CR, Liu SH, Das M, Shu CM. Evaluation for the thermokinetic of the autocatalytic reaction of cumene hydroperoxide mixed with phenol through isothermal approaches and simulations. Process Saf Environ Protect. 2018;117:426–38.
Chen KY, Wu SH, Wang YW, Shu CM. Runaway reaction and thermal hazards simulation of cumene hydroperoxide by DSC. J Loss Prev Process Ind. 2008;21:101–9.
Koltunov KY, Sobolev VI. Efficient cleavage of cumene hydroperoxide over HUSY zeolites: the role of Brønsted acidity. Appl Catal A Gen. 2008;336:29–34.
Duh YS, Kao CS, Hwang HH, Lee WL. Thermal decomposition kinetics of cumene hydroperoxide. Process Saf Environ Protect. 1998;76:271–6.
Stoessel F. What is your thermal risk. ChemEng Progress. 1993;10:68–75.
Wang YW, Shu CM, Duh YS, Kao CS. Thermal runaway hazards of cumene hydroperoxide with contaminants. IndEngChem Res. 2001;40:1125–32.
Liu SH, Shu CM, Hou HY. Application of thermal hazard analyses on process safety assessments. J Loss Prev Process Ind. 2015;33:59–69.
Townsend DI, Tou JC. Thermal hazard evaluation by an accelerating rate calorimetry. Thermochim Acta. 1980;37:1–30.
Andreozzi R, Marotta R, Sanchirico R. Thermal decomposition of acetic anhydride-nitric acid mixtures. J Hazard Mater. 2002;90(2):111–21.
Acknowledgements
The authors would like to express their sincere thanks to the Anhui University of Science and Technology in China under Contract Number QN201613 as well as to the Anhui Province Education Department, Natural Sciences Key Fund, China (Grant No. KJ2017A078).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, SH., Yu, CF. & Das, M. Thermal hazardous evaluation of autocatalytic reaction of cumene hydroperoxide alone and mixed with products under isothermal and non-isothermal conditions. J Therm Anal Calorim 140, 2325–2336 (2020). https://doi.org/10.1007/s10973-019-09017-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-09017-7