Abstract
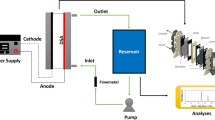
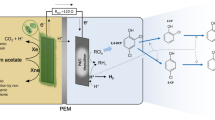
The objective of this work was to develop a mathematical model of an electrochemical flow reactor for the degradation of 2-chlorophenol. The reactor operates in batch recirculation and undivided mode under mass transport control and under galvanostatic conditions. The mathematical model proposed here was simulated on COMSOL Multiphysic® 5.3 software (involving the continuity and Navier-Stokes equation in a laminar regime, and the diffusion-convection equation with reaction term) interacting with the MATLAB® version R 2017a software (continuous stirred tank). The electrolysis process was carried out at a current density of 0.14 A m−2, a liquid flow rate of 1 L min−1 and pH = 7.3. The main results show that the mathematical model proposed here is in a very good agreement with the experimental study (correlation coefficient of 0.9917 and a reduced root-mean-square error of 0.4041). The final concentration of 2-chlorophenol estimated by the mathematical model was 0.0013 mol m−3, while the experimental concentration reached was 0.0001 mol m−3, confirming the predictive capacity of the mathematical model, as well as the efficiency of the electrochemical process implemented.

ᅟ









Similar content being viewed by others
Abbreviations
- u :
-
Liquid flow velocity, m s−1
- \( {D}_H=\frac{4{A}_{Cross}}{P_{Wet}} \) :
-
Equivalent hydraulic diameter of the rectangular flow channel, m
- A Cross = W ch × (S ch − S e):
-
Area of cross section, m2
- P Wet = 2W ch + 2(S ch − S e):
-
Wet perimeter, m
- W ch :
-
Channel width, m
- S ch :
-
Channel thickness, m
- S e :
-
Electrode thickness, m
- V T :
-
Tank volume, L
- Q :
-
Liquid flow rate, L min−1
- j :
-
Current density, A cm2
- C 2 − CPh :
-
Concentration of 2-chlorophenol, mol m−3
- C 2 − CPh, 0 :
-
Initial concentration of 2-chlorophenol, mol m−3
- \( {C}_{2- CPh}^{\mathrm{exp}} \) :
-
2-CPh concentrations of experiment, mol m−3
- \( {C}_{2- CPh}^{theor} \) :
-
2-CPh concentrations of theoretical model, mol m−3
- pH:
-
Logarithmic scale of acidity or basicity, dimensionless
- •OH:
-
Hydroxyl radical
- pK a :
-
Negative base-10 logarithm of the acid dissociation constant
- \( {K}_{ow}^a \) :
-
Octanol–water partition coefficient, dimensionless
- k :
-
Apparent first-order reaction rate constant, h−1
- -r i :
-
Reaction rate model, mol m−3 h−1
- C 0 :
-
Outlet concentration of the 2-CPh from tank that feeds the reactor, mol m−3
- t :
-
Time, h
- D i :
-
Diffusion coefficient, m2 s−1
- P :
-
Pressure, Pa
- P init :
-
Initial pressure, Pa
- P hydro :
-
Hydrodynamic pressure, Pa
- Ι :
-
Unit momentum vector, dimensionless
- F :
-
Volume force, N m−3
- \( \tilde{n} \) :
-
Unit normal vector, dimensionless
- g :
-
Gravity acceleration constant, m s−2
- n :
-
Number of data points
- N i :
-
Molar flux, mol h−1 m−2
- R 2 :
-
Correlation coefficient
- ν :
-
Kinematic viscosity of the fluid, m2 s−1
- ρ :
-
Density of the fluid, kg L−1
- μ :
-
Dynamic viscosity of the fluid, kg m−1 s−1
- ∇ :
-
Gradient
- \( \operatorname{Re}=\frac{u{D}_H}{\nu } \) :
-
Reynolds number, dimensionless
- UHPLC :
-
Ultrahigh-performance liquid chromatography
- BDD:
-
Boron-doped diamond
- 2-CPh :
-
2-chlorophenol
- DSA:
-
Dimensionally stable anode
- RTD:
-
Residence time distribution
- CST:
-
Continuous stirred tank
- RMSE:
-
Reduced root-mean-square error
- CFD:
-
Computational fluid dynamics
References
Wongwisate P, Chavadej S, Gulari E, Sreethawong T, Rangsunvigit P (2011) Effects of monometallic and bimetallic Au–Ag supported on sol–gel TiO2 on photocatalytic degradation of 4-chlorophenol and its intermediates. Desalination 272(1):154–163. https://doi.org/10.1016/j.desal.2011.01.016
Zhou L-C, Meng X-G, Fu J-W, Yang Y-C, Yang P, Mi C (2014) Highly efficient adsorption of chlorophenols onto chemically modified chitosan. Appl Surf Sci 292:735–741. https://doi.org/10.1016/j.apsusc.2013.12.041
Xu J, Lv X, Li J, Li Y, Shen L, Zhou H, Xu X (2012) Simultaneous adsorption and dechlorination of 2,4-dichlorophenol by Pd/Fe nanoparticles with multi-walled carbon nanotube support. J Hazard Mater 225-226:36–45. https://doi.org/10.1016/j.jhazmat.2012.04.061
Arellano-González MÁ, González I, Texier A-C (2016) Mineralization of 2-chlorophenol by sequential electrochemical reductive dechlorination and biological processes. J Hazard Mater 314:181–187. https://doi.org/10.1016/j.jhazmat.2016.04.048
Rubín E, Rodríguez P, Herrero R, Sastre de Vicente Manuel E (2006) Biosorption of phenolic compounds by the brown alga Sargassum muticum. J Chem Technol Biotechnol 81(7):1093–1099. https://doi.org/10.1002/jctb.1430
Kao P-C, Tzeng J-H, Huang T-L (2000) Removal of chlorophenols from aqueous solution by fly ash. J Hazard Mater 76(2):237–249. https://doi.org/10.1016/S0304-3894(00)00201-6
Lin Y-H (2017) Adsorption and biodegradation of 2-chlorophenol by mixed culture using activated carbon as a supporting medium-reactor performance and model verification. Appl Water Sci 7(7):3741–3757. https://doi.org/10.1007/s13201-016-0522-0
Rashid J, Barakat MA, Ruzmanova Y, Chianese A (2015) Fe3O4/SiO2/TiO2 nanoparticles for photocatalytic degradation of 2-chlorophenol in simulated wastewater. Environ Sci Pollut Res 22(4):3149–3157. https://doi.org/10.1007/s11356-014-3598-9
Ba-Abbad MM, Takriff MS, Kadhum AAH, Mohamad AB, Benamor A, Mohammad AW (2017) Solar photocatalytic degradation of 2-chlorophenol with ZnO nanoparticles: optimisation with D-optimal design and study of intermediate mechanisms. Environ Sci and Pollut Res 24(3):2804–2819. https://doi.org/10.1007/s11356-016-8033-y
Ananpattarachai J, Seraphin S, Kajitvichyanukul P (2016) Formation of hydroxyl radicals and kinetic study of 2-chlorophenol photocatalytic oxidation using C-doped TiO2, N-doped TiO2, and C,N co-doped TiO2 under visible light. Environ Sci Pollut Res 23(4):3884–3896. https://doi.org/10.1007/s11356-015-5570-8
Xu J, Wang F, Liu W, Cao W (2013) Nanocrystalline N-doped powders: mild hydrothermal synthesis and photocatalytic degradation of phenol under visible light irradiation. Int J Photoenergy 2013:1–7. https://doi.org/10.1155/2013/616139
Yu X, Zhou M, Hu Y, Groenen Serrano K, Yu F (2014) Recent updates on electrochemical degradation of bio-refractory organic pollutants using BDD anode: a mini review. Environ Sci Pollut Res 21(14):8417–8431. https://doi.org/10.1007/s11356-014-2820-0
Sirés I, Brillas E, Oturan MA, Rodrigo MA, Panizza M (2014) Electrochemical advanced oxidation processes: today and tomorrow. A review. Environ Sci Pollut Res 21(14):8336–8367. https://doi.org/10.1007/s11356-014-2783-1
Peralta E, Ruíz M, Martínez G, Mentado-Morales J, Zárate LG, Cordero ME, Garcia-Morales MA, Natividad R, Regalado-Méndez A (2018) Degradation of 4-Chlorophenol in a batch electrochemical reactor using BDD electrodes. Int J Eletrochem Sci 13(5):4625–4639. https://doi.org/10.20964/2018.05.21
Oturan MA (2014) Electrochemical advanced oxidation technologies for removal of organic pollutants from water. Environ Sci Pollut Res 21(14):8333–8335. https://doi.org/10.1007/s11356-014-2841-8
Rivero EP, Rivera FF, Cruz-Díaz MR, Mayen E, González I (2012) Numerical simulation of mass transport in a filter press type electrochemical reactor FM01-LC: comparison of predicted and experimental mass transfer coefficient. Chem Eng Res Des 90(11):1969–1978. https://doi.org/10.1016/j.cherd.2012.04.010
Vázquez L, Alvarez-Gallegos A, Sierra FZ, de León CP, Walsh FC (2013) CFD evaluation of internal manifold effects on mass transport distribution in a laboratory filter-press flow cell. J of Appl Electrochem 43(4):453–465. https://doi.org/10.1007/s10800-013-0530-9
Rivera Fernando F, Rodríguez Francisca A, Rivero Eligio P, Cruz-Díaz Martín R (2018) Parametric mathematical modelling of cristal violet dye electrochemical oxidation using a flow electrochemical reactor with BDD and DSA anodes in sulfate media. ijcre 16:In press. https://doi.org/10.1515/ijcre-2017-0116
Rivero EP, Ortega A, Cruz-Díaz MR, González I (2018) Modelling the transport of ions and electrochemical regeneration of the resin in a hybrid ion exchange/electrodialysis process for as(V) removal. J Appl Electrochem 48:597–610. https://doi.org/10.1007/s10800-018-1191-5
Rivera FF, Cruz-Díaz MR, Rivero EP, González I (2010) Analysis and interpretation of residence time distribution experimental curves in FM01-LC reactor using axial dispersion and plug dispersion exchange models with closed–closed boundary conditions. Electrochim Acta 56(1):361–371. https://doi.org/10.1016/j.electacta.2010.08.069
Regalado-Méndez A, Mentado-Morales J, Vázquez Carlos E, Martínez-Villa G, Cordero Mario E, Zárate Luis G, Skogestad S, Peralta-Reyes E (2018) Modeling and hydraulic characterization of a filter-press-type electrochemical reactor by using residence time distribution analysis and hydraulic indices. ijcre 16:In press. https://doi.org/10.1515/ijcre-2017-0210
Mascia M, Vacca A, Palmas S, Polcaro A (2007) Kinetics of the electrochemical oxidation of organic compounds at BDD anodes: modelling of surface reactions. J Appl Electrochem 37(1):71–76. https://doi.org/10.1007/s10800-006-9217-9
Mascia M, Vacca A, Polcaro AM, Palmas S, Ruiz JR, Da Pozzo A (2010) Electrochemical treatment of phenolic waters in presence of chloride with boron-doped diamond (BDD) anodes: experimental study and mathematical model. J Hazard Mater 174(1):314–322. https://doi.org/10.1016/j.jhazmat.2009.09.053
Scialdone O (2009) Electrochemical oxidation of organic pollutants in water at metal oxide electrodes: a simple theoretical model including direct and indirect oxidation processes at the anodic surface. Electrochim Acta 54(26):6140–6147. https://doi.org/10.1016/j.electacta.2009.05.066
Mascia M, Vacca A, Palmas S (2012) Fixed bed reactors with three dimensional electrodes for electrochemical treatment of waters for disinfection. Chem Eng J 211-212:479–487. https://doi.org/10.1016/j.cej.2012.09.091
Cruz-Díaz MR, Rivero EP, Rodríguez FA, Domínguez-Bautista R (2018) Experimental study and mathematical modeling of the electrochemical degradation of dyeing wastewaters in presence of chloride ion with dimensional stable anodes (DSA) of expanded meshes in a FM01-LC reactor. Electrochim Acta 260:726–737. https://doi.org/10.1016/j.electacta.2017.12.025
Rivero EP, Rodríguez FA, Cruz-Díaz MR, González I (2018) Reactive diffusion migration layer and mass transfer wall function to model active chlorine generation in a filter press type electrochemical reactor for organic pollutant degradation. Chem Eng Res Des. https://doi.org/10.1016/j.cherd.2018.07.010
Catellanos-Cruz M, Reyes EP, Cordero ME, Uribe-López JS, Mentado-Morales J, Zárate LG, Martínez-Villa G, Torres-Zárate SR, Regalado-Méndez A (2017) Mineralization of 2-Chlorophenol in a filterpress type electrochemical reactor: variable effects of flow rate, initial pH, and current density. Paper presented at the 10th world congress of chemical engineering. Barcelona, Spain, p 2017
Santana-Martínez G, Roa-Morales G, Martin del Campo E, Romero R, Frontana-Uribe BA, Natividad R (2016) Electro-Fenton and electro-Fenton-like with in situ electrogeneration of H2O2 and catalyst applied to 4-chlorophenol mineralization. Electrochim Acta 195:246–256. https://doi.org/10.1016/j.electacta.2016.02.093
Pérez T, León MI, Nava JL (2013) Numerical simulation of current distribution along the boron-doped diamond anode of a filter-press-type FM01-LC reactor during the oxidation of water. J Electroanal Chem 707:1–6. https://doi.org/10.1016/j.jelechem.2013.08.014
Sirés I, Brillas E (2012) Remediation of water pollution caused by pharmaceutical residues based on electrochemical separation and degradation technologies: a review. Environ Int 40:212–229. https://doi.org/10.1016/j.envint.2011.07.012
Rivera FF, CPd L, Walsh FC, Nava JL (2015) The reaction environment in a filter-press laboratory reactor: the FM01-LC flow cell. Electrochim Acta 161:436–452. https://doi.org/10.1016/j.electacta.2015.02.161
Mott HV, Green ZA (2015) On Danckwerts’ boundary conditions for the plug-flow with dispersion/reaction model. Chem Eng Commun 202(6):739–745. https://doi.org/10.1080/00986445.2013.871708
Sandoval MA, Fuentes R, Walsh FC, Nava JL, de León CP (2016) Computational fluid dynamics simulations of single-phase flow in a filter-press flow reactor having a stack of three cells. Electrochim Acta 216:490–498. https://doi.org/10.1016/j.electacta.2016.09.045
Brown CJ, Pletcher D, Walsh FC, Hammond JK, Robinson D (1993) Studies of space-averaged mass transport in the FM01-LC laboratory electrolyser. J Appl Electrochem 23(1):38–43. https://doi.org/10.1007/bf00241573
Vázquez L, Alvarez-Gallegos A, Sierra FZ, Ponce de León C, Walsh FC (2010) Simulation of velocity profiles in a laboratory electrolyser using computational fluid dynamics. Electrochim Acta 55(10):3437–3445. https://doi.org/10.1016/j.electacta.2009.08.066
Kim S, Kim Y-K (2004) Apparent desorption kinetics of phenol in organic solvents from spent activated carbon saturated with phenol. Cheml Eng J 98(3):237–243. https://doi.org/10.1016/j.cej.2003.10.006
Comninellis C (1994) Electrocatalysis in the electrochemical conversion/combustion of organic pollutants for waste water treatment. Electrochim Acta 39(11):1857–1862. https://doi.org/10.1016/0013-4686(94)85175-1
Sulaymon AH, Abbar AH (2012) Scale-up of electrochemical reactors. Electrolysis IntechOpen. https://doi.org/10.5772/48728
Walsh FC, Ponce de León C (2018) Progress in electrochemical flow reactors for laboratory and pilot scale processing. Electrochim Acta 280:121–148. https://doi.org/10.1016/j.electacta.2018.05.027
Acknowledgements
The authors are thankful for the support of the Programa para el Desarrollo Profesional Docente (PRODEP), [Project DSA/103.5/16/10242 with CUP: 2II1605, 2016]. We also wish to thank Ph.D. Aitor Aizpuru for checking the text.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PNG 16 kb)
Rights and permissions
About this article
Cite this article
Regalado-Méndez, A., Cruz-López, A., Mentado-Morales, J. et al. Mathematical modeling of the electrochemical degradation of 2-chlorophenol using an electrochemical flow reactor equipped with BDD electrodes. J Flow Chem 9, 59–71 (2019). https://doi.org/10.1007/s41981-018-00027-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41981-018-00027-4