Abstract
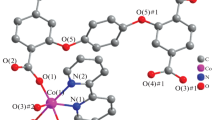
Two new metal–organic coordination polymer frameworks formulated as, {[Co(nta)]·H3O+}n (1), and {[Zn(ina)Cl2]·H3O+·H2O}n (2) (nta = nitriliotriacetic acid, ina = isonicotinate), have been synthesized under mild conditions and characterized by elemental analyses, IR spectrum, PXRD, thermal gravimetric analysis (TG) and single-crystal X-ray diffraction. Complex 1 crystallizes in the orthorhombic space group P2(1)2(1)2(1) with unit cell parameters a = 8.403(4) Å, b = 10.165(5) Å, c = 11.230 Å, β = 90, V = 959.3(9) Å3. Complex 2 crystallizes in the monoclinic space group P2(1)/c with unit cell parameters a = 7.1334(13) Å, b = 22.369(4) Å, c = 7.0089(13) Å, β = 101.099(2), V = 1097.5(3) Å3. In fact, 1 is a new 3D cobalt complex with the nta ligands acted as bridges to form network polymer. Each nta ligand bonds to three cobalt ions, and each cobalt ion coordinates with three nta ligands in different directions. 2 displays a new 1D zigzag chain linked by ina ligand and an interesting 1D hydrogen-bond infinite chain consists of seven-membered rings and four-membered rings. These chains are further linked to generate a new 3D framework.
Graphical Abstract
Two novel metal–organic coordination polymer frameworks have been synthesized under mild conditions and characterized by elemental analyses, IR spectrum, PXRD, thermal gravimetric analysis (TG) and single-crystal X-ray diffraction.






Similar content being viewed by others
References
Batten SR, Robson R (1998) Angew Chem Int Ed 37:1460
Leininger S, Olenyuk B, Stang PJ (2000) Chem Rev 100:853
Swiegers GF, Malefetse TJ (2000) Chem Rev 100:3483
Moulton B, Zaworotko MJ (2001) Chem Rev 101:1629
Holliday BJ, Mirkin CA (2001) Angew Chem Int Ed 40:2022
Qin L, Zhang Z, Zheng ZP, Speldrich M, Kögerler P, Xue W, Wang BY, Chen XM, Zheng YZ (2015) Dalton Trans 44:1456
Guez-Martın YR, Hernandez-Molina M, Delgado FS, Pasan J, Ruiz-Perez C, Sanchiz J, Lloret F, Julve M (2002) CrystEngComm 4:522
Zhang Z, Yin XH, Liu JZ, Su F, Gao P, Xiao Q (2011) J Chem Crystallogr 41:1839
Hu FL, Yin XH, Mi Y, Zhang JL, Zhuang Y, Dai XZ (2009) Inorg Chem Commun 12:628
Jenkins DM, Bernhard S (2010) Inorg Chem 49:11297
Li JR, Kuppler RJ, Zhou HC (2009) Chem Soc Rev 38:1477
Erre LS, Garribba E, Micera G, Pusino A, Sanna D (1997) Inorg Chim Acta 255:215
Jesus RN, Ribeiro MA, Inoue MH, Nunes FH, Samulewski RB (2014) J Chem Crystallogr 44:506
Zhao YP, Zhao CC, Xu TT, Huang Q, Du ZY (2014) J Chem Crystallogr 44:480
Colson SD, Dunning TH (1994) Science 265:43
Zhang Z, Yin XH (2013) Russ J Coord Chem 39:450
Barbour LJ, Orr GW, Atwood JL (2000) Chem Commun 10:859
Zhang Z, Wang YL, Yang GY (2017) Inorg Chem Commun 85:32
Barnard NI, Visser HG (2012) Inorg Chem Commun 15:40
White LS, Nolsson PV, Pignolet LH, Que LJ (1984) J Am Chem Soc 106:8312
Infantes L, Motherwell S (2002) CrystEngComm 4:454
Hu FL, Yin XH, Mi Y, Zhang JL, Zhuang Y, Dai XZ (2009) Inorg Chem Commun 12:628
Mir MH, Vittal JJ (2007) Angew Chem Int Ed 46:5925
Ghosh SK, Bharadwaj PK (2005) Eur J Inorg Chem 44:4886
Barbour LJ, Orr GW, Atwood JL (1998) Nature 393:671
Sreenivasulu B, Vittal JJ (2004) Angew Chem Int Ed 43:5893
Cheruzel LE, Pometun MS, Cecil MR, Mashuta MS, Wittebort RJ, Buchanan RM (2003) Angew Chem Int Ed 42:5452
Sheldrick GM (1996) SADABS. Program for Empirical Absorption Correction of Area Detector. University of Göttingen, Göttingen
Sheldrick GM (1997) SHELXTL V5.1 Software Reference Manual. Bruker AXS Inc., Madison
Visser HG, Purcell W, Basson SS (2001) Polyhedron 20:185
Okamoto K, Hidaka J, Fukagawa M, KanamoriI K (1992) Acta Cryst C48:1025
Barnard NI, Visser HG (2012) Inorg Chem Commun 15:40
Acknowledgements
This work was supported by the Research Fund for the Doctoral Program of Daqing Normal University (NO.16ZR04).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xue, H., Xu, HS., He, DF. et al. Synthesis, Crystal Structure and Thermal Property of Two New Coordination Polymers with Ligands Containing Both N and O Donors. J Chem Crystallogr 50, 133–138 (2020). https://doi.org/10.1007/s10870-019-00785-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-019-00785-6