Abstract
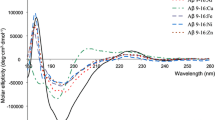
Zinc ions and glycosaminoglycans (GAGs) are found in amyloid deposits and are known to modulate the β-amyloid peptide (Аβ) aggregation, which is thought to be a key event in the pathogenesis of Alzheimer’s disease (AD). Correlation spectroscopy was used to study how the H6R and D7H mutations of the metal-binding domain (MBD) of Аβ42 affect the modulation of its zinc-induced aggregation by the model GAG heparin. The H6R mutation was shown to decrease and the D7H mutation to increase the Аβ42 propensity to aggregate in the presence of zinc ions. In addition, H6R diminished and D7H enhanced the modulating effect of heparin. The difference in the heparin-dependent modulation was associated with coordination of zinc ions within the MBDs of the mutant peptides. The findings indicate that anion-binding sites formed by complexes of zinc ions with the Аβ MBD play an essential role in the interaction of zinc-induced Аβ aggregates with heparin.




Similar content being viewed by others
REFERENCES
Haass C., Selkoe D.J. 2007. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell. Biol. 8, 101–112.
Faller P., Hureau C., Berthoumieu O. 2013. Role of metal ions in the self-assembly of the Alzheimer’s amyloid-β peptide. Inorg. Chem. 52, 12193–12206.
Kulikova A.A., Makarov A.A., Kozin S.A. 2015. Roles of zinc ions and structural polymorphism of β-amyloid in the development of Alzheimer’s disease. Mol. Biol. (Moscow). 49 (2), 217–230.
Lovell M.A., Robertson J.D., Teesdale W.J., Campbell J.L., Markesbery W.R. 1998. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 158, 47–52.
Miller L.M., Wang Q., Telivala T.P., Smith R.J., Lanzirotti A., Miklossy J. 2006. Synchrotron-based infrared and x-ray imaging shows focalized accumulation of Cu and Zn co-localized with β-amyloid deposits in Alzheimer’s disease. J. Struct. Biol.155, 30–37.
Suprun E.V., Radko S.P., Kozin S.A., Mitkevich V.A., Makarov A.A. 2018. Electrochemical detection of Zn(II)-induced amyloid-β aggregation: Insights into aggregation mechanisms. J. Electroanal. Chem.830–831, 34–42.
O’Callaghan P., Sandwall E., Li J.P., Yu H., Ravid R., Guan Z.Z., van Kuppevelt T.H., Nilsson L.N., Ingelsson M., Hyman B.T., Kalimo H., Lindahl U., Lannfelt L., Zhang X. 2008. Heparan sulfate accumulation with Aβ deposits in Alzheimer’s disease and Tg2576 mice is contributed by glial cells. Brain Pathol. 18, 548–561.
Bruinsma I.B., te Riet L., Gevers T., ten Dam G.B., van Kuppevelt T.H., David G., Kusters B., de Waal R.M., Verbeek M.M. 2010. Sulfation of heparan sulfate associated with amyloid-β plaques in patients with Alzheimer’s disease. Acta Neuropathol. 119, 211–220.
Ariga T., Miyatake T., Yu R.K. 2010. Role of proteoglycans and glycosaminoglycans in the pathogenesis of Alzheimer’s disease and related disorders: Amyloidogenesis and therapeutic strategies—a review. J. Neurosci. Res. 88, 2303–2315.
Bergamaschini L., Rossi E., Vergani C., De Simoni M.G. 2009. Alzheimer’s disease: Another target for heparin therapy. Sci. World J.9, 891–908.
Bush A.I., Tanzi R.E. 2008. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 5, 421–432.
Yoshiike Y., Tanemura K., Murayama O., Akagi T., Murayama M., Sato S., Sun X., Tanaka N., Takashima A. 2001. New insights on how metals disrupt amyloid β‑aggregation and their effects on amyloid-β cytotoxicity. J. Biol. Chem. 276, 32293–32299.
Radko S.P., Khmeleva S.A., Mantsyzov A.B., Kiseleva Y.Y., Mitkevich V.A., Kozin S.A., Makarov A.A. 2018. Heparin modulates the kinetics of zinc-induced aggregation of amyloid-β peptides. J. Alzheimers Dis. 63, 539–550.
Janssen J.C., Beck J.A., Campbell T.A., Dickinson A., Fox N.C., Harvey R.J., Houlden H., Rossor M.N., Collinge J. 2003. Early onset familial Alzheimer’s disease: Mutation frequency in 31 families. Neurology. 60, 235–239.
Chen W.T., Hong C.J., Lin Y.T., Chang W.H., Huang H.T., Liao J.Y., Chang Y.J., Hsieh Y.F., Cheng C.Y., Liu H.C., Chen Y.R., Cheng I.H. 2012. Amyloid-β (Aβ) D7H mutation increases oligomeric Aβ42 and alters properties of Aβ-zinc/copper assemblies. PLoS One. 7, e35807.
Khmeleva S.A., Mezentsev Yu.V., Kozin S.A., Mitke-vich V.A., Medvedev A.E., Ivanov A.S., Bodoev N.V., Makarov A.A., Radko S.P. 2015. Effect of mutations and modifications of amino acid residues on zinc-induced interaction of the metal-binding domain of β‑amyloid with DNA. Mol. Biol. (Moscow). 49 (3), 450–456.
Khmeleva S.A., Kozin S.A., Kiseleva Ya.Yu., Mitke-vich V.A., Makarov A.A., Radko S.P. 2016. Zinc-induced interactions of the metal-binding domain of beta-amyloid with nucleic acids and glycosaminoglycans. Mol. Biol. (Moscow). 50, 1049–1052.
Radko S.P., Khmeleva S.A., Suprun E.V., Kozin S.A., Bodoev N.V., Makarov A.A., Archakov A.I., Shumyantseva V.V. 2015. Physicochemical methods for studying β-amyloid aggregation. Biomed. Khim. 61, 203–218.
Suprun E.V., Khmeleva S.A., Kiseleva Y.Y., Radko S.P., Archakov A.I., Shumyantseva V.V. 2016. Quantitative aspects of electrochemical detection of amyloid-β aggregation. Electroanalysis. 28, 1977–1983.
Sabel C.E., Neureuther J.M., Siemann S. 2010. A spectrophotometric method for the determination of zinc, copper, and cobalt ions in metalloproteins using zincon. Anal. Biochem. 397, 218–226.
Istrate A.N., Kozin S.A., Zhokhov S.S., Mantsyzov A.B., Kechko O.I., Pastore A., Makarov A.A., Polshakov V.I. 2016. Interplay of histidine residues of the Alzheimer’s disease Aβ peptide governs its Zn-induced oligomerization. Sci. Rep. 6, 21734.
Olofsson A., Lindhagen-Persson M., Vestling M., Sauer-Eriksson A.E., Ohman A. 2009. Quenched hydrogen/deuterium exchange NMR characterization of amyloid-β peptide aggregates formed in the presence of Cu2+ or Zn2+. FEBS J.276, 4051–4060.
Lim K.H., Kim Y.K., Chang Y.T. 2007. Investigations of the molecular mechanism of metal-induced Aβ (1–40) amyloidogenesis. Biochemistry. 46, 13523–13532.
Zirah S., Kozin S.A., Mazur A.K., Blond A., Cheminant M., Segalas-Milazzo I., Debey P., Rebuffat S. 2006. Structural changes of region 1–16 of the Alzheimer disease amyloid β-peptide upon zinc binding and in vitro aging. J. Biol. Chem. 281, 2151–2161.
Tsvetkov P.O., Kulikova A.A., Golovin A.V., Tkachev Y.V., Archakov A.I., Kozin S.A., Makarov A.A. 2010. Minimal Zn2+ binding site of amyloid-β. Biophys. J.99, L84–L86.
Miller Y., Ma B., Nussinov R. 2012. Metal binding sites in amyloid oligomers: Complexes and mechanisms. Coord. Chem. Rev.256, 2245–2252.
Kozin S.A., Kulikova A.A., Istrate A.N., Tsvetkov P.O., Zhokhov S.S., Mezentsev Y.V., Kechko O.I., Ivanov A.S., Polshakov V.I., Makarov A.A. 2015. The English (H6R) familial Alzheimer’s disease mutation facilitates zinc-induced dimerization of the amyloid-β metal-binding domain. Metallomics. 7, 422–425.
Polshakov V.I., Mantsyzov A.B., Kozin S.A., Adzhubei A.A., Zhokhov S.S., van Beek W., Kulikova A.A., Indeykina M.I., Mitkevich V.A., Makarov A.A. 2017. A binuclear zinc interaction fold discovered in the homodimer of Alzheimer’s amyloid-β fragment with Taiwanese mutation D7H. Angew. Chem. Int. Ed. Engl. 56, 11734–11739.
ACKNOWLEDGMENTS
We used equipment of the Human Proteome Collective Access Center (Orekhovich Institute of Biomedical Chemistry).
Funding
This work was supported by the program “Basic Research for Biomedical Technologies” of the Presidium of the Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by T. Tkacheva
Abbreviations: AD, Alzheimer’s disease; GAG, glycosaminoglycan; Aβ, amyloid β; MBD, metal-binding domain; a.a., amino acid residue.
Rights and permissions
About this article
Cite this article
Radko, S.P., Khmeleva, S.A., Kiseleva, Y.Y. et al. Effects of the H6R and D7H Mutations on the Heparin-Dependent Modulation of Zinc-Induced Aggregation of Amyloid β. Mol Biol 53, 922–928 (2019). https://doi.org/10.1134/S0026893319060141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893319060141