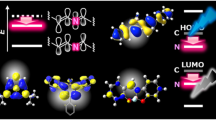
Abstract
The search for new fluorescent molecules for possible applications as functional π-electron systems and their conjugation with different nanomaterials is nowadays of paramount importance to broaden the availability of materials with different properties. Herein we present a diversity-oriented approach to heterocyclic luminophores based on a multicomponent Ugi reaction followed by a Pd-mediated cascade sequence. The new molecules are coupled to carbon nano-onions, and hybrid systems represent the first example of blue emitters conjugated with these carbon nanoparticles.

Similar content being viewed by others
References
Kroto H W, Heath J R, O’Brien S C, Curl R F, Smalley R E. C60: Buckminsterfullerene. Nature, 1985, 318(6042): 162–163
Ugarte D. Curling and closure of graphitic networks under electronbeam irradiation. Nature, 1992, 359(6397): 707–709
Ugarte D. Onion-like graphitic particles. Carbon, 1995, 33(7): 989–993
Mykhailiv O, Zubyk H, Plonska-Brzezinska M E. Carbon nanoonions: Unique carbon nanostructures with fascinating properties and their potential applications. Inorganica Chimica Acta, 2017, 468: 49–66
Palkar A, Melin F, Cardona C M, Elliott B, Naskar A K, Edie D D, Kumbhar A, Echegoyen L. Reactivity differences between carbon nano onions (cnos) prepared by different methods. Chemistry, an Asian Journal, 2007, 2(5): 625–633
Kuznetsov V L, Zilberberg I L, Butenko Y V, Chuvilin A L, Segall B. Theoretical study of the formation of closed curved graphite-like structures during annealing of diamond surface. Journal of Applied Physics, 1999, 86(2): 863–870
Sano N, Wang H, Alexandrou I, Chhowalla M, Teo K B K, Amaratunga G A J, Iimura K. Properties of carbon onions produced by an arc discharge in water. Journal of Applied Physics, 2002, 92 (5): 2783–2788
Alexandrou I, Wang H, Sano N, Amaratunga G A J. Structure of carbon onions and nanotubes formed by arc in liquids. Journal of Chemical Physics, 2004, 120(2): 1055–1058
Dorobantu D, Bota P M, Boerasu I, Bojin D, Enachescu M. Pulse laser ablation system for carbon nano-onions fabrication. Surface Engineering and Applied Electrochemistry, 2014, 50(5): 390–394
Chen X H, Deng F M, Wang J X, Yang H S, Wu G T, Zhang X B, Peng J C, Li W Z. New method of carbon onion growth by radiofrequency plasma-enhanced chemical vapor deposition. Chemical Physics Letters, 2001, 336(3): 201–204
Bartelmess J, Giordani S. Carbon nano-onions (multi-layer fullerenes): Chemistry and applications. Beilstein Journal of Nanotechnology, 2014, 5: 1980–1998
Georgakilas V, Guldi D M, Signorini R, Bozio R, Prato M. Organic functionalization and optical properties of carbon onions. Journal of the American Chemical Society, 2003, 125(47): 14268–14269
Liu Y, Vander Wal R L, Khabashesku V N. Functionalization of carbon nano-onions by direct fluorination. Chemistry of Materials, 2007, 19(4): 778–786
Rettenbacher A S, Perpall M W, Echegoyen L, Hudson J, Smith D W. Radical addition of a conjugated polymer to multilayer fullerenes (carbon nano-onions). Chemistry of Materials, 2007, 19 (6): 1411–1417
Cioffi C T, Palkar A, Melin F, Kumbhar A, Echegoyen L, Melle-Franco M, Zerbetto F, Rahman G M A, Ehli C, Sgobba V, Guldi D M, Prato M. A carbon nano-onion-ferrocene donor-acceptor system: Synthesis, characterization and properties. Chemistry (Weinheim an der Bergstrasse, Germany), 2009, 15(17): 4419–4427
Zhou L, Gao C, Zhu D, Xu W, Chen F F, Palkar A, Echegoyen L, Kong E S W. Facile functionalization of multilayer fullerenes (carbon nano-onions) by nitrene chemistry and “grafting from” strategy. Chemistry (Weinheim an der Bergstrasse, Germany), 2009, 15(6): 1389–1396
Flavin K, Chaur M N, Echegoyen L, Giordani S. Functionalization of multilayer fullerenes (carbon nano-onions) using diazonium compounds and “click” chemistry. Organic Letters, 2010, 12(4): 840–843
Tomita S, Fujii M, Hayashi S, Yamamoto K. Electron energy-loss spectroscopy of carbon onions. Chemical Physics Letters, 1999, 305 (3): 225–229
Chhowalla M,Wang H, Sano N, Teo K B K, Lee S B, Amaratunga G A J. Carbon onions: Carriers of the 217.5 nm interstellar absorption feature. Physical Review Letters, 2003, 90(15): 155504
Sek S, Breczko J, Plonska-Brzezinska M E, Wilczewska A Z, Echegoyen L. STM-based molecular junction of carbon nano-onion. ChemPhysChem, 2013, 14(1): 96–100
Zeiger M, Jäckel N, Aslan M, Weingarth D, Presser V. Understanding structure and porosity of nanodiamond-derived carbon onions. Carbon, 2015, 84: 584–598
Shenderova O, Tyler T, Cunningham G, Ray M, Walsh J, Casulli M, Hens S, McGuire G, Kuznetsov V, Lipa S. Nanodiamond and onion-like carbon polymer nanocomposites. Diamond and Related Materials, 2007, 16(4): 1213–1217
Macutkevic J, Adomavicius R, Krotkus A, Seliuta D, Valusis G, Maksimenko S, Kuzhir P, Batrakov K, Kuznetsov V, Moseenkov S, Shenderova O, Okotrub A V, Langlet R, Lambin P. Terahertz probing of onion-like carbon-PMMA composite films. Diamond and Related Materials, 2008, 17(7): 1608–1612
Bartolome J P, Echegoyen L, Fragoso A. Reactive carbon nanoonion modified glassy carbon surfaces as DNA sensors for human papillomavirus oncogene detection with enhanced sensitivity. Analytical Chemistry, 2015, 87(13): 6744–6751
Maffeis V, McCourt R O, Petracca R, Laethem O, Camisasca A, Colavita P E, Giordani S, Scanlan E M. Photocatalytic initiation of radical thiol-ene reactions using carbon-B2O3 nanocomposites. ACS Applied Nano Materials, 2018, 1(8): 4120–4126
Zeiger M, Jäckel N, Mochalin V N, Presser V. Review: Carbon onions for electrochemical energy storage. Journal of Materials Chemistry. A, Materials for Energy and Sustainability, 2016, 4(9): 3172–3196
Zheng D, Yang G, Zheng Y, Fan P, Ji R, Huang J, Zhang W, Yu J. Carbon nano-onions as a functional dopant to modify hole transporting layers for improving stability and performance of planar perovskite solar cells. Electrochimica Acta, 2017, 247: 548–557
D’Amora M, Rodio M, Bartelmess J, Sancataldo G, Brescia R, Cella Zanacchi F, Diaspro A, Giordani S. Biocompatibility and biodistribution of functionalized carbon nano-onions (f-CNOs) in a vertebrate model. Scientific Reports, 2016, 6(1): 33923
D’Amora M, Camisasca A, Lettieri S, Giordani S. Toxicity assessment of carbon nanomaterials in zebrafish during development. Nanomaterials (Basel, Switzerland), 2017, 7(12): 414
Trusel M, Baldrighi M, Marotta R, Gatto F, Pesce M, Frasconi M, Catelani T, Papaleo F, Pompa P P, Tonini R, Giordani S. Internalization of carbon nano-onions by hippocampal cells preserves neuronal circuit function and recognition memory. ACS Applied Materials & Interfaces, 2018, 10(20): 16952–16963
Lettieri S, d’Amora M, Camisasca A, Diaspro A, Giordani S. Carbon nano-onions as fluorescent on/off modulated nanoprobes for diagnostics. Beilstein Journal of Nanotechnology, 2017, 8: 1878–1888
Müller T J J, Bunz U H F. Functional Organic Materials. Syntheses, Strategies, and Applications. Weinheim: Wiley-VCH, 2007
Arcudi F, Ðorđević L, Prato M. Rationally designed carbon nanodots towards pure white-light rmission. Angewandte Chemie International Edition, 2017, 56(15): 4170–4173
Frasconi M, Marotta R, Markey L, Flavin K, Spampinato V, Ceccone G, Echegoyen L, Scanlan E M, Giordani S. Multifunctionalized carbon nano-onions as imaging probes for cancer cells. Chemistry (Weinheim an der Bergstrasse, Germany), 2015, 21 (52): 19071–19080
Bartelmess J, Baldrighi M, Nardone V, Parisini E, Buck D, Echegoyen L, Giordani S. Synthesis and characterization of far-red/NIR-fluorescent BODIPY dyes, solid-state fluorescence, and application as fluorescent tags attached to carbon nano-onions. Chemistry (Weinheim an der Bergstrasse, Germany), 2015, 21(27): 9727–9732
Lettieri S, Camisasca A, d’Amora M, Diaspro A, Uchida T, Nakajima Y, Yanagisawa K, Maekawa T, Giordani S. Far-red fluorescent carbon nano-onions as a biocompatible platform for cellular imaging. RSC Advances, 2017, 7(72): 45676–45681
Liu Y, Kim D Y. Ultraviolet and blue emitting graphene quantum dots synthesized from carbon nano-onions and their comparison for metal ion sensing. Chemical Communications, 2015, 51(20): 4176–4179
Müllen K, Scherf U. Organic light-emitting diodes—synthesis, properties, and applications. Weinheim: Wiley-VCH, 2006
Zhu M, Yang C. Blue fluorescent emitters: Design tactics and applications in organic light-emitting diodes. Chemical Society Reviews, 2013, 42(12): 4963–4976
Kuma H, Hosokawa C. Blue fluorescent OLED materials and their application for high-performance devices. Science and Technology of Advanced Materials, 2014, 15(3): 34201
Yang X, Xu X, Zhou G. Recent advances of the emitters for high performance deep-blue organic light-emitting diodes. Journal of Materials Chemistry. C, Materials for Optical and Electronic Devices, 2015, 3(5): 913–944
Bui T T, Goubard F, Ibrahim-Ouali M, Gigmes D, Dumur F. Thermally activated delayed fluorescence emitters for deep blue organic light emitting diodes: A review of recent advances. Applied Sciences (Basel, Switzerland), 2018, 8(4): 494
Froehlich J D, Young R, Nakamura T, Ohmori Y, Li S, Mochizuki A, Lauters M, Jabbour G E. Synthesis of Multi-Functional POSS Emitters for OLED Applications. Chemistry of Materials, 2007, 19 (20): 4991–4997
Krujatz F, Hild O R, Fehse K, Jahnel M, Werner A, Bley T. Exploiting the potential of oled-based photo-organic sensors for biotechnological applications. Chemical Sciences Journal, 2016, 7 (3): 134
Cairo C W, Key J A, Sadek C M. Fluorescent small-molecule probes of biochemistry at the plasma membrane. Current Opinion in Chemical Biology, 2010, 14(1): 57–63
Hong Y, Häußler M, Lam J W Y, Li Z, Sin K K, Dong Y, Tong H, Liu J, Qin A, Renneberg R, Tang B Z. Label-free fluorescent probing of G-quadruplex formation and real-time monitoring of dna folding by a quaternized tetraphenylethene salt with aggregationinduced emission characteristics. Chemistry (Weinheim an der Bergstrasse, Germany), 2008, 14(21): 6428–6437
Kuznetsov V L, Chuvilin A L, Butenko Y V, Mal’kov I Y, Titov V M. Onion-like carbon from ultra-disperse diamond. Chemical Physics Letters, 1994, 222(4): 343–348
Frasconi M, Maffeis V, Bartelmess J, Giordani S. Highly surface functionalized carbon nano-onions for bright light bioimaging. Methods and Applications in Fluorescence, 2015, 3(4): 0044005
Moni L, Gers-Panther C F, Anselmo M, Müller T J J, Riva R. Highly convergent synthesis of intensively blue emissive furo[2,3-c] isoquinolines by a palladium-catalyzed cyclization cascade of unsaturated Ugi products. Chemistry (Weinheim an der Bergstrasse, Germany), 2016, 22(6): 2020–2031
Dömling A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chemical Reviews, 2006, 106(1): 17–89
Hu R, Leung N L C, Tang B Z. AIE macromolecules: Syntheses, structures and functionalities. Chemical Society Reviews, 2014, 43 (13): 4494–4562
Banfi L, Basso A, Giardini L, Riva R, Rocca V, Guanti G. Tandem Ugi MCR/Mitsunobu cyclization as a short, protecting-group-free route to benzoxazinones with four diversity points. European Journal of Organic Chemistry, 2010, 2011(1): 100–109
Söveges B, Imre T, Póti Á L, Sok P, Kele Z, Alexa A, Kele P, Németh K. Tracking down protein—protein interactions via a FRET-system using site-specific thiol-labeling. Organic & Biomolecular Chemistry, 2018, 16(32): 5756–5763
Bartelmess J, De Luca E, Signorelli A, Baldrighi M, Becce M, Brescia R, Nardone V, Parisini E, Echegoyen L, Pompa P P, et al. Boron dipyrromethene (BODIPY) functionalized carbon nanoonions for high resolution cellular imaging. Nanoscale, 2014, 6(22): 13761–13769
Giordani S, Bartelmess J, Frasconi M, Biondi I, Cheung S, Grossi M, Wu D, Echegoyen L, O’Shea D F. NIR fluorescence labelled carbon nano-onions: Synthesis, analysis and cellular imaging. Journal of Materials Chemistry. B, Materials for Biology and Medicine, 2014, 2(42): 7459–7463
Acknowledgements
Istituto Italiano di Tecnologia and the University of Genova are gratefully acknowledged for financial support. S.G. acknowledges the COST Action CA 15107 “Multi-Functional Nano-Carbon Composite Materials Network (MultiComp)”. The authors wish to thank Prof. Luis Echegoyen (UTEP) for supervising V.M. in the synthesis of pristine CNOs.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Maffeis, V., Moni, L., Di Stefano, D. et al. Diversity-oriented synthesis of blue emissive nitrogen heterocycles and their conjugation with carbon nano-onions. Front. Chem. Sci. Eng. 14, 76–89 (2020). https://doi.org/10.1007/s11705-019-1833-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-019-1833-0