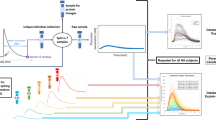
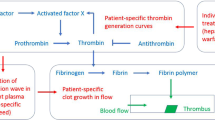
Abstract
Correct interpretation of the data from integral laboratory tests, including Thrombin Generation Test (TGT), requires biochemistry-based mathematical models of blood coagulation. The purpose of this study is to describe the experimental TGT data from healthy donors and hemophilia A (HA) and B (HB) patients. We derive a simplified ODE model and apply it to analyze the TGT data from healthy donors and HA/HB patients with in vitro added tissue factor pathway inhibitor (TFPI) antibody. This model allows the characterization of hemophilia patients in the space of three most important model parameters. The proposed approach may provide a new quantitative tool for the analysis of experimental TGT. Also, it gives a reduced model of coagulation verified against clinical data to be used in future theoretical large-scale modeling of thrombosis in flow.




Similar content being viewed by others
Abbreviations
- TGT:
-
Thrombin generation test
- TGC:
-
Thrombin generation curve
- PPP:
-
Platelet poor plasma
- TF :
-
Tissue factor
- X :
-
Factor X
- Xa :
-
Activated factor Xa. Idem for all factors
- ETP:
-
Endogenous thrombin potential, time-integral of thrombin concentration kinetics in TGT
- TFPI :
-
Tissue factor pathway inhibitor
- AB :
-
TFPI antibody
References
Belyaev AV, Dunster JL, Gibbins JM, Panteleev MA, Volpert V (2018) Modeling thrombosis in silico: frontiers, challenges, unresolved problems and milestones. Phys Life Rev. 26:57–95
Bouchnita A, Galochkina T, Kurbatova P, Nony P, Volpert V (2017) Conditions of microvessel occlusion for blood coagulation in flow. Int J Num Methods Biomed Eng 33:e2850
Chelle P, Morin C, Montmartin A, Piot M, Cournil M, Tardy-Poncet B (2018) Evaluation and calibration of in silico models of thrombin generation using experimental data from healthy and haemophilic subjects. Bull Math Biol 80:1989–2025
Chelle P, Montmartin A, Piot M, Cournil M, Lienhart A, Genre-Volot F, Chambost H, Morin C (2019) Tissue factor pathway inhibitor is the main determinant of thrombin generation in haemophilic patients. Haemophilia. 25(2):343–8
Chelle P, Montmartin A, Piot M, Cournil M, Morin C, Tardy-Poncet B (2017) Determinants of thrombin generation between haemophilic and healthy individuals. Blood (in press)
Chowdary P (2018) Inhibition of tissue factor pathway inhibitor (TFPI) as a treatment for haemophilia: rationale with focus on concizumab. Drugs. 78:881–890
Dargaud Y, Béguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, Hemker CH, Negrier C (2005) Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost 93(3):475–480
Dargaud Y, Negrier C, Rusen L et al (2018) Individual thrombin generation and spontaneous bleeding rate during personalized prophylaxis with Nuwiq® (human-cl rhFVIII) in previously treated patients with severe haemophilia A. J Haemophilia. 24:619–627. https://doi.org/10.1111/hae.13493
Dunster J, King J (2017) Mathematical modelling of thrombin generation: asymptotic analysis and pathway characterization. IMA J Appl Math 82:60–96
Galochkina T, Bouchnita A, Kurbatova P, Volpert V (2017) Reaction-diffusion waves of blood coagulation. Math Biosci 288:130–139
Galochkina T, Ouzzane H, Bouchnita A, Volpert V (2017) Traveling wave solutions in the mathematical model of blood coagulation. Appl Anal 96(16):2891–2905
Gissel M, Orfeo T, Foley JH, Butenas S (2012) Effect of BAX499 aptamer on tissue factor pathway inhibitor function and thrombin generation in models of hemophilia. Thromb Res 130:948–955
Goutelle S, Maurin M, Rougier F, Barbaut X, Bourguignon L, Ducher M, Maire P (2008) The Hill equation: a review of its capabilities in pharmacological modelling. Fundam Clin Pharmacol 22:633–648
Hemker HC et al (2002) The Calibrated Automated Thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb 32(5–6):249–53
Hemker H Coenraad et al (2003) Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb 33(1):4–15
Hemker HC, Béguin S (1995) Thrombin generation in plasma: its assessment via the endogenous thrombin potential. Thromb Haemost 74(1):134–38
Hill AV (1910) The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol (Lond). 40:4–7
Hockin MF, Jones KC, Everse SJ, Mann KG (2002) A model for the stoichiometric regulation of blood coagulation. J Biol Chem 277:18322–18333
Hoffman M, Meng ZH, Roberts HR, Monroe DM (2005) Rethinking the coagulation cascade. Jap J Thromb Hemost 16(1):70–81
Krasotkina YV, Sinauridze EI, Ataullakhanov F (2000) Spatiotemporal dynamics of fibrin formation and spreading of active thrombin entering non-recalcified plasma by diffusion. Biochim Biophys Acta G Gen Subj 1474(3):337–45
Lancé MD (2015) A general review of major global coagulation assays: thrombelastography, thrombin generation test and clot waveform analysis. Thromb J 13(1):1
Mann KG, Butenas S, Brummel K (2003) Dyn Thromb Format. Arteriosclerosis, thrombosis, and vascular biology 23(1):17–25
Orfeo T, Butenas S, Brummel-Ziedins KE, Mann KG (2005) The tissue factor requirement in blood coagulation. J Biol Chem 280:42887–42896
Panteleev MA, Ovanesov MV, Kireev DA, Shibeko AM, Sinauridze EI, Ananyeva NM, Butylin AA, Saenko EL, Ataullakhanov FI (2006) Spatial propagation and localization of blood coagulation are regulated by intrinsic and protein C pathways, respectively. Biophys J 90:1489–1500
Panteleev MA, Balandina AN, Lipets EN, Ovanesov MV, Ataullakhanov FI (2010) Task-oriented modular decomposition of biological networks: trigger mechanism in blood coagulation. Biophys J 98:1751–1761
Parunov LA, Fadeeva OA, Balandina AN, Soshitova NP, Kopylov KG, Kumskova MA, Gilbert JC, Schaub RG, Mcginness KE, Ataullakhanov FI, Panteleev MA (2011) Improvement of spatial fibrin formation by the anti-TFPI aptamer BAX499: changing clot size by targeting extrinsic pathway initiation. J Thromb Haemost 9:1825–1834
Parunov LA, Soshitova NP, Fadeeva OA, Balandina AN, Kopylov KG, Kumskova MA, Gilbert JC, Schaub RG, McGinness KE, Ataullakhanov FI, Panteleev MA (2014) Drug-drug interaction of the anti-TFPI aptamer BAX499 and factor VIII: Studies of spatial dynamics of fibrin clot formation in hemophilia A. Thromb Res. 133:112–119
Tokarev A, Krasotkina YuV, Ovanesov MV, Panteleev MA, Azhigirova MA, Volpert VA, Ataullakhanov FI, Butilin AA (2006) Spatial dynamics of contact-activated fibrin clot formation in vitro and in silico in haemophilia. B: Effects of severity and ahemphil b treatment. Math Model Nat Phenom 1(2):124–137
Tokarev A, Ratto N, Volpert V (2019) Mathematical modeling of thrombin generation and wave propagation: from simple to complex models and backwards. In: BIOMAT international symposium on mathematical and computational biology (in press)
Trossaërt M, Regnault V, Sigaud M, Boisseau P, Fressinaud E, Lecompte T (2008) Mild hemophilia A with factor VIII assay discrepancy : using thrombin generation assay to assess the bleeding phenotype. J Thromb Haemost 6(3):486–493
Van’t Veer C, Mann KG (1997) Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin-III, and heparin cofactor-II. J Biol Chem 272(7):4367–4377
Wagenvoord RJ, Hemker PW, Hemker HC (2006) The limits of simulation of the clotting system. J Thromb Haemost 4:1331–38
Waters EK, Sigh J, Friedrich U, Hilden I, Sørensen BB (2017) Concizumab, an anti-tissue factor pathway inhibitor antibody, induces increased thrombin generation in plasma from haemophilia patients and healthy subjects measured by the thrombin generation assay. Haemophilia. 23:769–776
Acknowledgements
This work was partially supported by the “RUDN University Program 5-100” to A.T. and V.V. The authors are grateful to the anonymous referees for useful suggestions that allowed us to improve the results and the presentation of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
1.1 From the Simulating to the Reduced Model
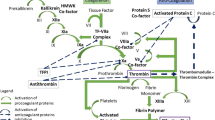
The coagulation cascade explained in Sect. 2.1 can be described using Fig. 5. The equations corresponding to this model give the system (1–17).
In the following approximations, we will explicitly refer to one of the Hypothesis of Sect. 2.2. The new constants will not always be explicitly expressed from the previous ones, since we do not use this expressions.
The first Hypothesis A1 leads to the equality: \(u_i+v_i=v_i^0\). Hence, \(v_i=v_i^0-u_i\). In order to describe the action of anticoagulant factors, we only apply this approximation to the inactive factors V, VIII, IX, X and XI.
Equations (3, 5, 7, 9) and (11), respectively, become as follows:
We will now use the fact that some reactions have high kinetics rates, and rapidly reach their equilibrium. Following assumption A2, we set the time derivative equal to zero. We first proceed with Eq. (17) and we get the equality:
It is applied to (14), and using, as before, the Hypothesis A2 of high kinetic rates, we obtain:
Similarly, we have from Eqs. (15) and (16):
These two approximations lead to some simplifications: (32) simplifies equations (26) and (27) for activated factors VIIIa and IXa, while (33) simplifies (25) for activated factor Va. Using Hypothesis A2 we have the following expressions for activated factors Va, VIIIa, IXa and XIa:
Let us now simplify these four equations using the third Hypothesis A3 according to which anticoagulating factors are in excess and their concentrations are higher than the concentrations of other factors. Thus, the denominators of (34–37) can be approximated by constants. Moreover, since \(\kappa _1 \gg \sigma _5\) Chelle et al. (2018), it can be assumed that \(\kappa _1 TF v_7 \gg \sigma _5 TFPI u_{10}\). Therefore, we can write (31) in the following form:
Hence, we have the following equalities:
Let us now focus our attention on Eq. (28) for activated factor Xa. Considering Eqs. (32, 33, 38, 40) and (41), we obtain:
Equation (43) can be considered as always valid in our approximation, but once the initiation phase is over, this expression can be simplified assuming two hypothesis: the reaction has reached its equilibrium (Hypothesis A2) and the concentration of anticoagulant factors is high (Hypothesis A3). In this case we have the following expression for activated factor Xa:
Finally, we deal with Eqs. (1) and (2) for thrombin and prothrombin. In this reaction, the activation of thrombin happens through the terms \(k_2^1u_{10}P\) and \(k_2^2c_3P\). The first one is due to the quantity of activated factor Xa produced after the initiation while the second one is the impact of the positive feedback loop in the amplification phase. Thus, it is possible to approximate the value of \(c_3\) using Eqs. (33, 39) and (44). We obtain the following equations:
Under Hypothesis A3, we assume that the concentrations of anticoagulating factors are in excess, and for further simplification we assume that the concentration of antithrombin is constant. To find this constant, we use the approximation of \(c_1\) given by (31). In this case we obtain:
The concentration of antithrombin is very large and the coagulation only uses some part of it (from 3400 nM/L at the beginning to 2100 nM/L at the end). Furthermore, this anticoagulation effect only appears after the amplification phase where the concentration of activated factor Xa is stabilized and remains constant. Since we are expressing the anticoagulating factor, we preserve the dependence on TFPI, IX and XI, so we obtain:
Finally, we apply the equality (48) to the three remaining ODEs (43, 45, 46), and we obtain:
Using the notation
we obtain the Reduced model in the following form:
We note that coefficients \(K_i\) depend on the combinations of parameters leading to possible correlations between them. For example, the correlation of the coefficients \(K_2\), \(K_3\), \(K_7\), and \(K_8\) can be observed under the variation of \(v_9^0\) for all other parameters are fixed. This is the case of hemophilia patients for whom the value of \(v_9^0\) is less than for normal subjects.
1.2 Table of the Features Values
Table presents the characterization of TGC for healthy and hemophilia subjects. The data are obtained with added TFPI antibodies (set 1) or factors VIII, IX (set 2).
Mean | Min | Max | |
|---|---|---|---|
Healthy | |||
ETP | 584.0292 | 281.365 | 1009.341 |
Max | 42.3619 | 16.7496 | 84.5972 |
TtP | 13.8375 | 9.39 | 19.67 |
Lag | 5.899 | 3.38 | 12 |
Tt0 | 37.448 | 32.33 | 49.79 |
Hemophilia, set 1 | |||
ETP | 317.0233 | 89.6358 | 788.2092 |
Max | 18.6827 | 3.6511 | 82.1361 |
TtP | 11.7627 | 6 | 25.33 |
Lag | 2.5818 | 0.59 | 5.98 |
Tt0 | 37.4717 | 24.67 | 49.67 |
Hemophilia, set 2 | |||
ETP | 249.9358 | 45.1653 | 645.3389 |
Max | 10.9127 | 1.8174 | 33.965 |
TtP | 29.4 | 13.33 | 58.67 |
Lag | 9.6667 | 1.33 | 26.33 |
Tt0 | 48.844 | 41 | 58.67 |
1.3 Smoothing Algorithm
The exponential smoothing algorithm for the sequence \((x_n)_{n\ge 0}\) is defined by the formula:
where \(\alpha \in [0,1]\). We write: \((y_n)=S((x_n))\). The new sequence \((y_n)_{n\ge 0}\) contains the smoothing of the sequence \((x_n)_{n\ge 0}\). The value of \(\alpha\) can depends on n. In our case, \(x_n\) is the measure of thrombin concentration at time n. We write N, the number of measures. For our database we mostly preserve the peak of thrombin, and mainly smooth the tail of the TGC. Hence, \(\alpha\) is close to 1 until the tail start, and then \(\alpha\) decreases. We choose two different values for \(\alpha\). Considering a data with N measures, we have \(\alpha =0.9\) for \(n=0\) to N / 3 (the beginning of the tail), and \(\alpha = 0.2\) afterwards. These values where chosen empirically in order to always have a positive concentration of thrombin, which was not always the case in the initial experiment data. The smoothing algorithm is applied twice on the data: the final data used is given by \(S(S(x_n))\).
1.4 Fitting for Hemophilia Patients
A typical case of fitting of the data with model (21–23) is shown in Fig. 6
1.5 Validation of the Reduced model
The three models are plotted in Fig. 7, and the relative errors for ETP, maximum of thrombin, lag time and time to maximum, for models (1–17) and (21–23) compared to the Hockin model, are under 10%. Figure 8 shows how the main features of TGC evolve as the concentration of TFPI varies. It can be seen that these dependencies are similar for all three models, though the time to maximum for model (21–23) increases faster than for the other two models. Parameters of the Hockin model for Fig. 8 are chosen using the values in Chelle et al. (2018). They correspond to a virtual healthy subject. Then, parameters for models (1–17) and (21–23) are found fitting their respective TGC to the TGC obtained with the Hockin model.
1.6 The Hill Equation (Goutelle et al. 2008; Hill 1910)
We use the Hill equation to determine the concentration of TFPI when TFPI antibodies are added. More generally, the Hill equation is used to represent the binding of ligands (here, the antibodies) to macromolecules (here, the TFPI). The chemical reactions writes:

where P is the macromolecule, L the ligand, \(PL_n\) the product and n the coefficient of Hill. Considering the affinity constant, \(K_a\):
and \(\theta\) the fraction of the bounded protein, the Hill equation writes:
The coefficient of the Hill equation represents the cooperativity between different subunits of a ligand. Here, this coefficient is chosen equal to 1 since there is no cooperativity between the several subunits of a TFPI antibody. It means that the affinity of TFPI for a antibody does not depend on whether or not other TFPI molecules are already bound to that antibody.
Hence, the Hill equation provides the following equation for the evolution of TFPI:
where \([TFPI]_0\) is the initial concentration of TFPI while \([TFPI_{AB}]\) is the concentration of TFPI antibodies.
In the model, the variation of TFPI impacts the coefficient \(k_9\), which corresponds to the anticoagulant part. Constant \(K_a\) was first determined for several patients, then the median value was chosen since \(K_a\) should not dependent on the subject.
1.7 Correlation Between ETP and Maximum of Thrombin
Figure 9 shows that there is a linear correlation between ETP and the maximum of thrombin for healthy and hemophilia patients in the experimental thrombin generation curves.
Rights and permissions
About this article
Cite this article
Ratto, N., Tokarev, A., Chelle, P. et al. Clustering of Thrombin Generation Test Data Using a Reduced Mathematical Model of Blood Coagulation. Acta Biotheor 68, 21–43 (2020). https://doi.org/10.1007/s10441-019-09372-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-019-09372-w