Abstract
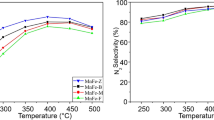
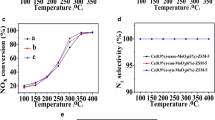
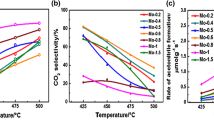
Molybdenum-exchanged ZSM-5 catalysts were tested in ethane ammoxidation into acetonitrile at 500 °C and at a very low contact time (0.08 s). The solids were prepared by sublimation, impregnation in CCl4 and solid-state ion exchange methods. The hydration state of the zeolite strongly affected the nature of MoCl5 and Mo(CO)6 decomposition products and, therefore, the concentration of stabilized Mo species in the final catalysts. In effect, using dehydrated ZSM-5 zeolite, the sublimation of MoCl5 led to the most active catalyst (TOF = 8.78 s−1) due to the presence, essentially, of [MoO4]2− (77%) and [Mo2O7]2− (10%) besides less-active crystalline MoO3 (12%) and traces of heptamers. However, the impregnation and the solid-state ion exchange of MoCl5 as well as the sublimation of Mo(CO)6 led to less-active catalysts owing to the presence of inefficient MoO3 oxide phase. In fact, moderate concentrations of crystalline MoO3 should coexist with [MoO4]2− species in order to activate C2H6 into C2H4 instead of enhancing the deep hydrocarbons’ oxidation.











Similar content being viewed by others
References
Global acetonitrile market to reach 143 Kilotons by 2024 (CAGR 4%). https://www.abnewswire.com/pressreleases/global-acetonitrile-market-to-reach-143-kilotons-by-2024-cagr-4_400673.html. Accessed 26 June 2019
O.B. Rudakov, L.V. Rudakova, V.F. Selemenev, Acetonitrile as tops solvent for liquid chromatography and extraction. J. Anal. Chromatogr. Spectrosc. 1, 1–19 (2018). https://doi.org/10.24294/jacs.v1i2.883
M.M. Miller, A.J. DelMonte, Chapter 6.2: Six-membered ring systems: diazines and benzo derivatives. Prog. Heterocycl. Chem. 23, 371–402 (2011). https://doi.org/10.1016/B978-0-08-096805-6.00013-9
J.-F. Cote, D. Brouillette, J.E. Desnoyers, J.-F. Rouleau, J.-M. St-Arnaud, G. Perron, Dielectric constants of acetonitrile, gamma-butyrolactone, propylene carbonate, and 1,2-dimethoxyethane as a function of pressure and temperature. J. Solut. Chem. 25, 1163–1173 (1996). https://doi.org/10.1007/BF00972644
N.D. Tring, D. Lepage, D. Aymé-Perrot, A. Badia, M. Dollé, D. Rochefort, An artificial lithium protective layer that enables the use of acetonitrile-based electrolytes in lithium metal batteries. Angew. Chem. Int. Ed. 57, 5072–5075 (2018). https://doi.org/10.1002/anie.201801737
E.L. Tollefson, R.M. Decker, C.B. Johnson, Development of a process for production of acetonitrile from acetic acid and ammonia. Can. J. Chem. Eng. 48, 219–223 (1970). https://doi.org/10.1002/cjce.5450480223
A. Tripodi, E. Bahadori, D. Cespi, F. Passarini, F. Cavani, T. Tabanelli, I. Rossetti, Acetonitrile from bioethanol ammoxidation: process design from the grass-roots and life cycle analysis. ACS Sustain. Chem. Eng. 6, 5441–5451 (2018). https://doi.org/10.1021/acssuschemeng.8b00215
F. Ayari, M. Mhamdi, J. Alvarez-Rodriguez, A.R. Guerrero-Ruiz, G. Delahay, A. Ghorbel, Cr-ZSM-5 catalysts for ethylene ammoxidation: effects of precursor nature and Cr/Al molar ratio on the physicochemical and catalytic properties. Microporous Mesoporous Mater. 171, 166–178 (2013). https://doi.org/10.1016/j.micromeso.2012.12.026
E. Mannei, F. Ayari, C. Petitto, E. Asedegbega-Nieto, A.R. Guerrero-Ruiz, G. Delahay, M. Mhamdi, A. Ghorbel, Light hydrocarbons ammoxidation into acetonitrile over Mo-ZSM-5 catalysts: effect of molybdenum precursor. Microporous Mesoporous Mater. 241, 246–257 (2017). https://doi.org/10.1016/j.micromeso.2016.12.021
F. Veatch, J. L. Callahan, E. C. Milberger, R. W. Foreman, in Proceedings of the 2nd International Congress on Catalysis, New York, 1960, vol. 2, p. 2647
J.F. Brazdil, M.A. Toft, Encyclopedia of Catalysis, Ammoxidation (Wiley, Hoboken, 2010), pp. 1–62. https://doi.org/10.1002/0471227617.eoc020
S. Essid, F. Ayari, R. Bulánek, J. Vaculík, M. Mhamdi, G. Delahay, A. Ghorbel, Over- and low-exchanged Co/BEA catalysts: general characterization and catalytic behaviour in ethane ammoxidation. Catal. Today 304, 103–111 (2018). https://doi.org/10.1016/j.cattod.2017.08.027
S. Essid, F. Ayari, R. Bulánek, J. Vaculík, M. Mhamdi, G. Delahay, A. Ghorbel, Improvement of the conventional preparation methods in Co/BEA zeolites: characterization and ethane ammoxidation. Solid State Sci. 93, 13–23 (2019). https://doi.org/10.1016/j.solidstatesciences.2019.04.008
E. Mannei, F. Ayari, E. Asedegbega-Nieto, M. Mhamdi, G. Delahay, Z. Ksibi, A. Ghorbel, Physicochemical and catalytic properties of over- and low-exchanged Mo-ZSM-5 ammoxidation catalysts. Chem. Paper 73, 619–633 (2019). https://doi.org/10.1007/s11696-018-0617-1
F. Solymosi, A. Cserenyi, A. Szoke, T. Bansagi, A. Oszko, Aromatization of methane over supported and unsupported Mo-based catalysts. J. Catal. 165, 150–161 (1997). https://doi.org/10.1006/jcat.1997.1478
J.R. Johns, R.F. Howe, Preparation of molybdenum mordenite from MoCl5. Zeolites 5, 251–256 (1985). https://doi.org/10.1016/0144-2449(85)90096-X
R.F. Howe, J. Ming, W.S. Tin, Z.J. Hua, Comparison of zeolites and aluminophosphates as hosts for transition metal complexes. Catal. Today 6, 113–122 (1989). https://doi.org/10.1016/0920-5861(89)85013-8
Y. Xu, S. Liu, X. Guo, L. Wang, M. Xie, Methane activation without using oxidants over Mo/HZSM-5 zeolite catalysts. Catal. Lett. 30, 135–149 (1994). https://doi.org/10.1007/BF00813680
D. Wang, J.H. Lunsford, M.P. Rosynek, Characterization of a Mo/ZSM-5 catalyst for the conversion of methane to benzene. J. Catal. 169, 347–358 (1997). https://doi.org/10.1006/jcat.1997.1712
G. Dantsin, K.S. Suslick, Sonochemical preparation of a nanostructured bifunctional catalyst. J. Am. Chem. Soc. 122, 5212–5214 (2000). https://doi.org/10.1021/ja994300w
A.K. Galway, Melting and thermal decompositions of solids. An appraisal of mechanistic interpretations of thermal processes in crystals. J. Therm. Anal. Calorim. 87, 601–615 (2007). https://doi.org/10.1007/s10973-006-7529-y
F. Ayari, E. Mannei, E. Asedegbega-Nieto, M. Mhamdi, A.R. Guerrero-Ruiz, G. Delahay, A. Ghorbel, Elucidation of the solid-state ion exchange mechanism of MoCl5 into ZSM-5 zeolite. Thermochim. Acta 655, 269–277 (2017). https://doi.org/10.1016/j.tca.2017.07.011
J.J. Cruywagen, Protonation, oligomerization, and condensation reactions of vanadate (V), molybdate (VI), and tungstate (VI). Adv. Inorg. Chem. 49, 127–182 (2000). https://doi.org/10.1016/S0898-8838(08)60270-6
F. Ayari, E. Mannei, E. Asedegbega-Nieto, M. Mhamdi, A.R. Guerrero-Ruiz, G. Delahay, A. Ghorbel, Solid–state ion exchange of ammonium heptamolybdate tetrahydrate into ZSM-5 zeolite. J. Therm. Anal. Calorim. 131, 1295–1306 (2018). https://doi.org/10.1007/s10973-017-6545-4
S.D. Djajanti, R.F. Howe, MOCVD in Zeolites using Mo(CO)6 and W(CO)6 as precursors. Stud. Surf. Sci. Catal. 97, 197–204 (1995). https://doi.org/10.1016/S0167-2991(06)81890-2
J.-H. Park, T.S. Sudarshan, Chemical Vapor Deposition, vol. 2, Surface engineering series (ASM International, Cleveland, 2001), p. 3
A. Antiñolo, P. Cañizares, F.C. Hermosilla, J.F. Baeza, F.J. Fúnez, A. de Lucas, A. Otero, L. Rodríguez, J.L. Valverde, A grafted methane partial oxidation catalyst from MoO2(acac)2 and HZSM-5 zeolite. Appl. Catal. A: Gen. 193, 139–146 (2000). https://doi.org/10.1016/S0926-860X(99)00423-8
Q. Guo, L. Li, L. Chen, Y. Wang, S. Ren, B. Shen, Benzylation of anisole catalyzed by MoCl5 or MoCl5/molecular sieve system. Energy Fuels 23, 51–54 (2009). https://doi.org/10.1021/ef800680p
E. Mannei, F. Ayari, E. Asedegbega-Nieto, M. Mhamdi, A.R. Guerrero-Ruiz, G. Delahay, A. Ghorbel, Solid-state ion exchange of molybdenum (VI) acetylacetonate into ZSM-5 zeolite. Thermochim. Acta 652, 150–159 (2017). https://doi.org/10.1016/j.tca.2017.03.020
F. Ayari, E. Mannei, E. Asedegbega-Nieto, M. Mhamdi, A.R. Guerrero-Ruiz, G. Delahay, A. Ghorbel, More insight on the isothermal spreading of solid MoO3 into ZSM-5 zeolite. React. Kinet. Mech. Catal. 124, 419–436 (2018). https://doi.org/10.1007/s11144-018-1357-5
Y. Song, C. Sun, W. Shen, L. Lin, Hydrothermal post–synthesis of HZSM-5 zeolite to enhance the coke–resistance of Mo/HZSM-5 catalyst for methane dehydroaromatization reaction: reconstruction of pore structure and modification of acidity. Appl. Catal. A: Gen. 317, 266–274 (2007). https://doi.org/10.1016/j.apcata.2006.10.037
R.W. Borry, Y.H. Kim, A. Huffsmith, J.A. Reimer, E. Iglesia, Structure and density of Mo and acid sites in Mo-exchanged H-ZSM5 catalysts for nonoxidative methane conversion. J. Phys. Chem. B 103, 5787–5796 (1999). https://doi.org/10.1021/jp990866v
E. Oldfield, J. Haase, K.D. Schmitt, S.E. Schramm, Characterization of zeolites and amorphous silica-aluminas by means of aluminum-27 nuclear magnetic resonance spectroscopy: a multifield, multiparameter investigation. Zeolites 14, 101–109 (1994). https://doi.org/10.1016/0144-2449(94)90003-5
E. Lippmaa, A. Samoson, M. Mägi, High-resolution aluminum–27 NMR of aluminosilicates. J. Am. Chem. Soc. 108, 1730–1735 (1986). https://doi.org/10.1021/ja00268a002
M.B. Rao, R.G. Jenkins, Molecular dimensions and kinetic diameters for diffusion for various species. Carbon 25, 445–446 (1987). https://doi.org/10.1016/0008-6223(87)90018-2
F.S. Xiao, S. Zheng, J. Sun, R. Yu, S. Qiu, R. Xu, Dispersion of inorganic salts into zeolites and their pore modification. J. Catal. 176, 474–487 (1998). https://doi.org/10.1006/jcat.1998.2054
C.A. Fyfe, G.J. Kennedy, C.T. De Schutter, G.T. Kokotailo, Sorbate-induced structural changes in ZSM-5 (silicalite). J. Chem. Soc. Chem. Commun. (1984). https://doi.org/10.1039/C39840000541
Y. Marcus, The Properties of Solvents, vol. 4 (Wiley, England, 1999), p. 239
D.C. Baertsch, H.H. Funke, J.L. Falconer, R.D. Noble, Permeation of aromatic hydrocarbon vapors through silicalite–zeolite membranes. J. Phys. Chem. 100, 7676–7679 (1996). https://doi.org/10.1021/jp960226h
K.G. Marek, K. Tarach, M. Choi, 2,6-di-tert-butylpyridine sorption approach to quantify the external acidity in hierarchical zeolites. J. Phys. Chem. C 118, 12266–12274 (2014). https://doi.org/10.1021/jp501928k
J. Goetze, I. Yarulina, J. Gascon, F. Kapteijn, B.M. Weckhuysen, Revealing lattice expansion of small-pore zeolite catalysts during the methanol-to-olefins process using combined Operando X-ray diffraction and UV–Vis spectroscopy. ACS Catal. 8, 2060–2070 (2018). https://doi.org/10.1021/acscatal.7b04129
M. Niwa, M. Iwamoto, K. Segawa, Temperature-programmed desorption of ammonia on zeolites. Influence of the experimental conditions on the acidity measurement. Bull. Chem. Soc. Jpn. 59, 3735–3739 (1986). https://doi.org/10.1246/bcsj.59.3735
P. Sarv, C. Fernadez, J.-P. Amoureux, K. Keskinen, Distribution of tetrahedral aluminium sites in ZSM-5 type zeolites: an 27Al (Multiquantum) Magic Angle Spinning NMR Study. J. Phys. Chem. 100, 19223–19226 (1996). https://doi.org/10.1021/jp962519g
G.A. Khan, C.A. Hogarth, Optical absorption spectra of evaporated V2O5 and co-evaporated V2O5/B2O3 thin films. J. Mater. Sci. 26, 412–416 (1991). https://doi.org/10.1007/BF00576535
R.S. Weber, Effect of local structure on the UV-visible absorption edges of molybdenum oxide clusters and supported molybdenum oxides. J. Catal. 151, 470–474 (1995). https://doi.org/10.1006/jcat.1995.1052
L. Čapek, J. Dědeček, P. Sazama, B. Wichterlová, The decisive role of the distribution of Al in the framework of beta zeolites on the structure and activity of Co ion species in propane-SCR-NOx in the presence of water vapour. J. Catal. 272, 44–54 (2010). https://doi.org/10.1016/j.jcat.2010.03.013
D. Kaucký, A. Vondrová, J. Dědeček, B. Wichterlová, Activity of Co ion sites in ZSM-5, Ferrierite, and Mordenite in selective catalytic reduction of NO with methane. J. Catal. 194, 318–329 (2000). https://doi.org/10.1006/jcat.2000.2925
E. Mannei, F. Ayari, M. Mhamdi, M. Almohalla, A.R. Guerrero-Ruiz, G. Delahay, A. Ghorbel, Ammoxidation of C2 hydrocarbons over Mo-zeolite catalysts prepared by solid-state ion exchange: nature of molybdenum species. Microporous Mesoporous Mater. 219, 77–86 (2016). https://doi.org/10.1016/j.micromeso.2015.07.036
P. Krüger, M. Petukhov, B. Domenichini, A. Berkó, S. Bourgeois, Monolayer formation of molybdenum carbonyl on Cu(111) revealed by scanning tunneling microscopy and density functional theory. J. Phys. Chem. C 116, 10617–10622 (2012). https://doi.org/10.1021/jp300832a
Acknowledgements
This work is dedicated to the memory of Professor Farhat Farhat (Faculté de Pharmacie de Monastir, Tunisie), a great educator in the field of analytical chemistry and the memory of Professor Mohamed Salah Belkhiria (Faculté des Sciences de Monastir, Tunisie), a talented educator of a great knowledge in the field of coordination chemistry.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mannei, E., Ayari, F., Asedegbega-Nieto, E. et al. Catalytic behaviour of molybdenum-based zeolitic materials prepared by organic-medium impregnation and sublimation methods. J IRAN CHEM SOC 17, 1087–1101 (2020). https://doi.org/10.1007/s13738-019-01837-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01837-6