Abstract
A new method to prepare Cu/ZrO2 catalysts by reducing CuO/ZrO2 with hydrazine hydrate is reported, and the prepared catalysts were used to synthesize disodium iminodiacetate by diethanolamine dehydrogenation. Hydrazine hydrate can rapidly reduce the CuO/ZrO2 precursor powder in an alkaline environment at a moderate temperature. The ratio of Cu0/Cu+ at the Cu/ZrO2 surface was controlled by the amount of hydrazine hydrate and the reduction reaction time. The formation mechanism of disodium glycine as the main byproduct and iminodiacetate were deduced by investigating the product yield, the reaction time, and the presence of acetaldehyde in the evolved gas. It has been shown that the ratio of Cu0/Cu+ in Cu/ZrO2 significantly affects the dehydrogenation of diethanolamine into disodium iminodiacetate. Cu0 and Cu+ are the catalytic activity centers in the dehydrogenation of diethanolamine which respectively produce intermediate aldehydes and an ester via nucleophilic addition reactions. The formation mechanism of sodium glycinate is related to the tautomerism of intermediate products and Schiff base hydrolysis.
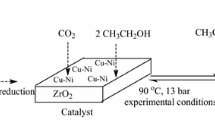
Graphic Abstract
The formation mechanism of disodium iminodiacetate and sodium glycine producing by the dehydrogenation of diethanolamine over the Cu/ZrO2 catalysts which were prepared by a new reduction method.













Similar content being viewed by others
References
Alibhai MF, Stallings WC (2001) Proc Natl Acad Sci USA 98:2944–2946
Chen D, Li J, Li GR, Chen BH, Yin FX (2013) Chem Ind Eng Prog 32:1635–1640
Andreev DV, Sergeev EE, Gribovskii AG, Makarshin LL, Prikhod’ko SA, Adonin NY, Pai ZP, Parmon VN (2017) Chem Eng J 330:899–905
Zheng XJ, Yang GW, Xu XX, Yang G (2001) Fine Chem 18:608–610
Wang Y, Zhao Y, Zhao Z, Lan X, Xu J, Xu W, Duan Z (2019) Acta Chim Sin 71:661–668
Hickman DA, Mosner K, Ringer JW (2015) Chem Eng J 278:447–453
Andreev DV, Gribovskii AG, Makarshin LL, Adonin NY, Prikhod’ko SA, Pai ZP, Parmon VN (2013) Catal Ind 5:1–8
Yang AS, Pan YF, Sun Q, Cheng R, Zheng YP, Xu GM (2010) J Chem Eng Chin Univ 4:590–595
Lu Z, Gao D, Yin H, Wang A, Liu S (2015) J Ind Eng Chem 31:301–308
Nagaiah P, Venkat Rao M, Thirupathaiah K, Venkateshwarlu V, David Raju B, Rama Rao KS (2018) Res Chem Intermed 44:5817–5831
Yin H, Yin H, Wang A, Shen L, Liu Y, Zheng Y (2017) J Nanosci Nanotechnol 17:1255–1266
Sun D, Misu T, Yamada Y, Sato S (2019) Appl Catal A. https://doi.org/10.1016/j.apcata.2019.06.007
Ohira M, Liu H, He D, Hirata Y, Sano M, Suzuki T, Miyake T (2018) J Jpn Petrol Inst 61:205–212
Gao D, Yin H, Wang A, Shen L, Liu S (2015) J Ind Eng Chem 26:322–332
Wang LX, Zhu WC, Zheng DF, Yu X, Cui J, Jia MJ, Zhang WX, Wang ZL (2010) React Kinet Mech Catal 101:365–375
Inui K, Kurabayashi T, Sato S (2002) J Catal 212:207–215
Inui K, Kurabayashi T, Sato S (2002) Appl Catal A 237:53–61
Ro I, Liu YF, Ball MR, Jackson DHK, Chada JP, Sener C, Kuech TF, Madon RJ, Huber GW, Dumesic JA (2016) ACS Catalysis 6:7040–7050
Zonetti PC, Celnik J, Letichevsky S, Gaspar AB, Appel LG (2011) J Mol Catal A 334:29–34
Sato AG, Volanti DP, Freitas IC, Longo El, Bueno JC (2012) Catal Commun 26:122–126
Bai GY, Wang YL, Li F, Zhao Z, Chen GF, Li N, Han X (2012) Catal Lett 143:101–107
Freitas IC, Damyanova S, Oliveira DC, Marques CMP, Bueno JMC (2014) J Mol Catal A 381:26–37
Chen CQ, Ruan CX, Zhan YY, Lin XY, Zheng Q, Wei KM (2014) Int J Hydrogen Energy 39:317–324
Ji DH, Zhu WC, Wang ZL, Wang GJ (2007) Catal Commun 8:1891–1895
Hu Q, Fan GL, Yan L, Cao XZ, Zhang P, Wang BY, Li F (2016) Green Chem 18:2317–2322
Shi QJ, Liu N, Liang Y (2007) Chin J Catal 28:57–61
Komandur VR, Guggilla VS, Chakravarthula SS, Vattikonda VR (2007) J Phys Chem B 11:543–550
Kim S (1974) J Electron Spectrosc Relat Phenom 3:217–226
Severino F, Brito JL, Laine J, Fierro JL, L´opez Agudo A (1998) J Catal 177:82–95
Acharyya SS, Ghosh S, Bal R (2014) ACS Sustain Chem Eng 2:584–589
Maiti S, Llorca J, Dominguez M, Colussi S, Trovarelli A, Priolkar KR, Aquilanti G, Gayen A (2016) J Power Sources 304:319–331
Unnikrishnan P, Srinivas D (2012) Ind Eng Chem Res 51:6356–6363
Hu Q, Yang L, Fan GL, Li F (2016) Chem Nano Mat 2:888–896
Wang J, Lei Z, Qin H, Zhang L, Li F (2011) Ind Eng Chem Res 50:7120–7128
Yang YC, Duan ZK, Liu WY, Li GL, Xiong Y (2001) Chem React Eng Technol 17:210–215
Balaraman E, Khaskin E, Leitus G, Milstein D (2013) Nat Chem 5:122–125
Zhang M, Zhao YJ, Liu Q, Yang L, Fan GL, Li F (2016) Dalton Trans 45:1093–1102
Wang ZY, Liu XY, Rooney DW, Hu P (2015) Surf Sci 640:181–189
Takeshita K, Nakamura S, Kawamoto K (1978) Bull Chem Soc Jpn 51:2622–2627
Neurock M, Tao ZY, Chemburkar A, Hibbitts DD, Iglesia E (2017) Faraday Discuss 197:59–86
Manrı́quez ME, López T, Gómez R, Navarrete J (2004) J Mol Catal A 220:229–237
Jiang ZW, Zhang ZR, Song JL, Meng QL, Zhou HC, He ZH, Han BX (2016) ACS Sustain Chem Eng 4:305–311
Acknowledgements
Support from the National Natural Science Foundation of China (Grant No. NSFC 21576229) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhu, H., Duan, Z. et al. Study on the Structure of Cu/ZrO2 Catalyst and the Formation Mechanism of Disodium Iminodiacetate and Sodium Glycine. Catal Lett 150, 1111–1120 (2020). https://doi.org/10.1007/s10562-019-02989-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-019-02989-z