Abstract
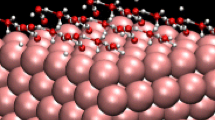
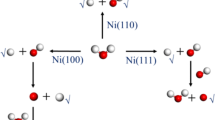
Water formation according to Had + OHad →H2O has recently been proposed as an intermediate step during hydrogen oxidation in alkaline solutions. Choosing Ni/Cu bimetallic surfaces as model catalysts, we have investigated the energetics and kinetics of this step in the form of a bifunctional mechanism. With the aid of density functional theory, we have identified several reaction paths on such surfaces with very low activation energies, suggesting that this step can be very fast. In all cases, the initial adsorption sites have both nickel and copper atoms as nearest neighbors. We suggest a strategy to find other bifunctional surfaces with good catalytic properties for this reaction.

A bifunctional mechanism for the formation of water as an intermediate step for hydrogen oxidation is investigated by density functional theory. Using copper/nickel alloys of various compositions as an example, potential energy surfaces are calculated for various reaction paths. On some favorable sites the reaction may be too fast to be observed by electrochemical methods.




Similar content being viewed by others
References
N. Danilovic, R. Subbaraman, D. Strmcnik, K.-C. Chang, A.P. Paulikas, V.R. Stamenkovic, N.M. Markovic, . Angew. Chem. Int. Ed. 51, 12495 (2012)
Y Wang, G Wang, G Li, B Huang, J Pan, Q Liu, J Han, L Xiao, J Lu, L Zhuang, . Energy Environ. Sci. 8, 177 (2015)
J Li, S Ghoshal, MK Bates, TE Miller, V Davies, E Stavitski, K Attenkofer, S Mukerjee, Z-F Ma, Q Jia, . Angew. Chem. Int. Ed. 129, 15880 (2017)
D. Strmcnik, M. Uchimura, C Wang, R Subbaraman, N. Danilovic, D. van der Vliet, A. P. Paulikas, V. R. Stamenkovic, N. M. Markovic, . Nat. Chem. 5, 300 (2013)
D. Salmazo, D. F. Juarez, A.G. Oshchepkov, O.V. Cherstiouk, A. Bonnefont, R.R. Natzmutdinov, W. Schmickler, E. Savinova, . Electrochim. Acta. 30, 452 (2019)
E.S. Davydova, S. Mukerjee, F. Jaouen, D. Dekel, . ACS Catal. 8, 6665 (2018)
M. Gong, D.Y. Wang, C.C. Chen, B.J. Hwang, H. Dai, . Nano Res. 9, 28 (2016)
P. Quaino, G. Belletti, A. Shermukhamedov, D.V. Glukhov, E. Santos, W. Schmickler, R. Nazmutdinov, . PhysChemChemPhys. 19, 26812 (2017)
J.K. Nørskov, T. Bligaard, A. Logadottir, J.R. Kitchin, J.G. Chen, S. Pandelov, U. Stimming, . J. Electrochem. Soc. 152, J23 (2005)
G.S. Karlberg, T.F. Jaramillo, E. Skulason, J. Rossmeisl, T. Bligaard, J.K. Nørskov, . Phys. Rev. Lett. 99, 126101 (2007)
J.K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J.R. Kitchin, T. Bligaard, H.-J. Jonsson, . Phys. Chem. B. 108, 17886 (2004)
E. Santos, P. Hindelang, P. Quaino, E.N. Schulz, G. Soldano, W. Schmickler, . Chemphyschem. 12, 2274 (2011)
F. Juarez, D. Salmazo, E.-R. Savinova, P. Quaino, G. Belletti, E. Santos, W. Schmickler, . J. Electroanal. Chem. 832, 137 (2019)
L. Pinto, P. Quaino, M. Arce, E. Santos, W. Schmickler, . ChemPhysChem. 15, 2003 (2014)
P. Quaino, F. Juarez, E. Santos, W. Schmickler, . Beilstein J. Nanotechnol. 5, 846 (2014)
E. Santos, P. Quaino, W. Schmickler, . Electrochim. Acta. 55, 4346 (2010)
O.V. Cherstiouk, P.A. Simonov, A.G. Oshchepkov, V.I. Zaikovskii, T. Kardash, A. Bonnefont, V.N. Parmon, E.R. Savinova, . J. Electroanal. Chem. 783, 146 (2016)
A.G. Oshchepkov, P.A. Simonov, O.V. Cherstiouk, R.R. Nazmutdinov, D.V. Glukhov, V.I. Zaikovskii, T.Y. Kardash, R.I. Kvon, A. Bonnefont, A.N. Simonov, V.N. Parmon, E.R. Savinova, . Top. Catal. 58, 1181 (2015)
G. Henkelman, H. Jónsson, . J. Chem. Phys. 113, 9978 (2000)
G. Henkelman, B. P. Uberuaga, H. Jónsson, . J. Chem. Phys. 113, 9901 (2000)
J.L.C. Fajín, M.N.D.S. Cordeiro, F. Illas, J.R.B. Gomes, . J. Catal. 276, 92 (2010)
J.L.C. Fajín, M.N.D.S. Cordeiro, F. Illas, J.R.B. Gomes, . J. Catal. 313, 24 (2014)
A. Mohsenzadeh, K. Bolton, T. Richards, . Surf. Sci. 62, 71 (2014)
A. Mohsenzadeh, T. Richards, K.B. Surf, . Science. 64, 53 (2016)
H. Ibach. Physics of Surfaces and Interfaces, chapter 10 (Springer, Berlin, 2006)
Z-D He, Y-X Chen, E. Santos, W. Schmickler, . Angew. Chem. Int. Ed. 57, 2 (2018)
J. J. Mortensen, L. B. Hansen, K. W. Jacobsen, . Phys Rev. B. 71, 035109 (2005)
J. P. Perdew, K. Burke, M. Ernzerhof, . Phys. Rev. Lett. 77, 3865 (1996)
Y. Pan, H. Zhang, D. Shi, J. Sun, S. Du, F. Liu, H. Gao, . Adv. Mater. 21, 2777 (2009)
H. J. Monkhorst, J. D. Pack, . Phys. Rev. B. 13, 5188 (1976)
Acknowledgments
All authors thank Elizabeth Santos from Ulm University for useful discussions.
Funding
This study was financially supported by the Deutsche Forschungsgemeinschaft (FOR 1376). W.S. thanks CONICET for continued support. A generous grant of computing time from the Baden-Württemberg grid is gratefully acknowledged. P.Q. thanks PICT-2014-1084, CONICET, and UNL for support. D. Salmazo thanks CNPq-Brazil (248817/2013-2) for a fellowship.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Technical Details
Appendix: Technical Details
DFT calculations were performed using the GPAW code [27]. Perdew Burke and Erzenhof (PBE) [28] exchange-correlation functional was combined with PAW [29] to solve KS equations. The wave-functions were expanded using real-space uniform grids (with 0.2 Å of spacing) and the finite-difference approximation. Brillouin-zone integration was done using a 4 × 4 × 1 k-point Monkhorst- Pack grid [30], which corresponds to a (2 × 2) surface unit cell. For relaxations, the two bottom layers were fixed at the calculated positions corresponding to the bulk, and coverage of 1/4 when there is only one adsorbate considered. In all the calculations, 20 Å of vacuum was considered. The geometry convergence was achieved when the total forces were less than 0.02 eV/Å. Spin polarization was considered.
Rights and permissions
About this article
Cite this article
Juarez, F., Salmazo, D., Quaino, P. et al. Hydrogen Oxidation in Alkaline Media: the Bifunctional Mechanism for Water Formation. Electrocatalysis 10, 584–590 (2019). https://doi.org/10.1007/s12678-019-00546-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-019-00546-1