Abstract
The high variability in the reproductive biology of stingless bees makes them very amenable for comparative studies with other eusocial bee taxa. We investigated the structural organization of the ovaries of Melipona quadrifasciata queens and workers kept under different social conditions by analyzing their general histology, mitotic activity, and microfilament organization. The overall dynamics of ovarian activity were similar in the two castes, and at emergence their ovarioles contained a previtellogenic follicle. Stingless bees and honey bees differ in the structural organization in the lower germarium, but they have in common synchronized mitotic activity and putative germ line stem cells in the terminal filament. Unlike honey bees, stingless bee workers lay trophic eggs in addition to reproductive eggs. The overall similarities in oogenesis between the two taxa suggest that the decision to form trophic eggs should only occur in the late stages of oogenesis.
Zusammenfassung
Die Tatsache, dass Stachellose Bienen nicht nur hocheusocial sind, sondern auch sehr variabel in ihrer Reproduktionsbiologie, macht sie zu interessanten Studienobjekten für vergleichende Untersuchungen, insbesondere mt der Honigbiene, Apis mellifera. In der vorliegenden Arbeit analysierten wir die strukturelle Organisation der Ovariolen und die Dynamik der Ovogenese bei der Stachellosen Biene Melipona quadrifasciata, in Königinnen und Arbeiterinnen unterschiedlichen Alters und unter verschiedenen sozialen Haltungsbedingungen.
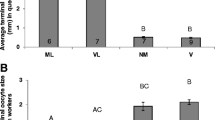
Im Hinblick auf die generelle Histologie der Ovariolen (Abb. 1–3) fanden wir nur wenige Unterschiede zwischen Königinnen und Arbeiterinnen, ausser dass erstere ein wesenlich längeres Germarium aufweisen, in dem sich eine grössere Zahl an Follikeln sequentiell entwickelt. Ein zweiter Unterschied liegt in der Zahl der prävitellogenen Follikel, die bereits beim Schlupf aus der Brutzelle angelegt sind. Während die Ovariolen von Arbeiterinnen lediglich einen prävitellogenen Follikel aufweisen, sind dies bei Königinnen stets mehrere. Diese Befunde stehen in Einklang mit der Eiproduktion junger M. quadrifascata Arbeiterinnen, die neben Nähreiern auch funktionelle Eier ablegen können, aus denen sich Männchen entwickeln.
Auch im Hinblick auf die Dynamik der Mitoseprozesse, die durch BrdU-Inkubation und Detektion in Ovariolen sichtbar gemacht wurden (Abb. 4), unterschieden sich Königinnen und Arbeiterinnen nur wenig. Sowohl im Terminalfilum als auch im Germarium zeigte sich eine regionale Synchronie im Zellzyklus, die sich im weiteren Verlauf der Oogenese sukzessiv verliert. In diesem Punkt sind sich Stachellose Bienen und Honigbienen sehr ähnlich. F-Aktin wurde mittels TRITC-Palloidin-Markierung und Laserkonfokalmikroskopie sichbar gemacht (Abb. 5). Bereits die frühesten Oogenesestadien wiesen F-Aktin in den Fusomen auf, das mit der Auflösung der verzweigten Polyfusome im weiteren Verlauf der Oogenese in die Ringkanäle übergeht. Erst mit der Differenzierung von Oocyten und Nährzellen im unteren Bereich des Germariums und dann nach Abtrennung der Follikel aus dem Germarium weisen die Nährzellen und insbesondere die Oocyten ein stark markiertes corticales Aktin-Cytoskelet auf.
Eine interessante Parallele in der Ovariolenstruktur von Honigbienen und Stachellosen Bienen bildet das Vorkommen eines zweiten Zelltyps im Terminalfilum. Interkaliert zwischen den typischen abgeplatteten somatischen Zellen, die wie in einem Münzstapel angeordnet sind, fanden sich auch bei M. quadrifasciata abgerundete Zellen, die ein nur schwach färbbares Cytoplasma und kein ausgeprägtes Aktin-Cytoskelett aufwiesen. Es könnte sich hierbei wie auch bei Honigbienen um Stammzellen der Keimbahn handeln.
Similar content being viewed by others
References
Amdam G.V., Csondes A., Fondrk M.K., Page R.E. (2006) Complex social behaviour derived from maternal reproductive traits, Nature 439, 76–78.
Beig D. (1972) The production of males in queenright colonies of Trigona (Scaptotrigona) postica, J. Apic. Res. 11, 33–39.
Boleli I.C., Simões Z.L.P., Bitondi M.M.G. (1999) Cell death in ovarioles causes permanent sterility in Frieseomelitta varia workers bees, J. Morphol. 242, 271–282.
Büning J. (1994) The Insect Ovary, Chapman & Hall, London.
Bylemans D., Borovsky D., Hunt D.F., Shabanowitz J., Grauwels L., De Loof A. (1994) Sequencing and characterization of trypsin modulating oostatic factor (TMOF) from the ovaries of the grey fleshfly, Neobellieria (Sarcophaga) bullata, Regul. Peptides 50, 61–72.
Cepeda O.I. (2006) Division of labor during brood production in stingless bees with special reference to individual participation, Apidologie 37, 175–190.
Chinh T.X., Grob G.B.J., Meeuwsen F., Sommeijer M.J. (2003) Patterns of male production in the stingless bee Melipona favosa (Apidae, Meliponini), Apidologie 34, 161–170.
Cooley L., Theurkauf W.E. (1994) Cytoskeletal functions during Drosophila oogenesis, Science 266, 590–596.
Cruz-Landim C. (2000) Ovarian development in Meliponine bees (Hymenoptera: Apidae) the effect of queen presence and food on worker ovary development and egg production, Genet. Mol. Biol. 23, 83–88.
Cruz-Landim C., Cruz Höfling M.A. (1971) Cytochemical and ultrastructural studies on eggs from workers and queens of Trigona, Rev. Bras. Pesq. Med. Biol. 4, 19–25.
De Loof A., Bylemans D., Schoofs L., Janssen I., Spittaels K., Vanden Broeck J., Huybrechts R., Borovsky D., Hua Y.J., Koolman J., Sower S. (1995) Folliculostatins, gonadotropins and a model for control of growth in the grey flesh-fly, Neobellieria (Sarcophaga) bullata, Insect Biochem. Moler. Biol. 25, 661–667.
Drummond P.M., Oldroyd B.P., Osborne K. (2000) Worker reproduction in Austroplebeia australis Friese (Hymenoptera, Apidae, Meliponini), Insect. Soc. 47, 333–336.
Engels W., Engels E. (1977) Vitellogenin und Fertilität bei Stachellosen Bienen, Insect. Soc. 24, 71–94.
Engels W., Imperatriz-Fonseca V.L. (1990) Caste development, reproductive strategies, and control of fertility in honey bees and stingless bees, in: Engels W. (Ed.), Social Insects — an evolutionary approach to castes and reproduction, Springer Verlag, Berlin, pp. 167–230.
Gutzeit H.O., Zissler D., Fleig R. (1993) Oogenesis in the honeybee Apis mellifera: cytological observations on the formation and differentiation of previtellogenic ovarian follicles, Roux’s Arch. Dev. Biol. 202, 181–191.
Hartfelder K., Steinbrück G. (1997) Germ cell cluster formation and cell death are alternatives in caste-specific differentiation of the larval honey bee ovary, Invertebr. Reprod. Dev. 31, 237–250.
Hartfelder K., Makert G.R., Judice C.C., Pereira G.A.G., Santana W.C., Dallacqua R., Bitondi M.M.G. (2006) Physiological and genetic mechanisms underlying caste development, reproduction and division of labor in stingless bees, Apidologie 37, 144–163.
Jablonska A., Bilinski S.M. (2001) Structure of ovarioles in adult queens and workers of the common wasp, Vespula germanica (Hymenoptera: Vespidae), Folia Biol. 49, 191–198.
Jablonska A., Kisiel E. (2002) Distribution of cytoskeletal elements in the ovarioles of Mutilla sp. (Insecta, Hymenoptera), Folia Histochem. Cyto. 40, 223–224.
Kawakita A., Ascher J.S., Sota T., Kato M., Roubik D.W. (2008) Phylogenetic analysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae), Apidologie 39, 163–175.
Koedam D., Cepeda-Aponte O.I., Imperatriz-Fonseca V.L. (2007) Egg laying and oophagy by reproductive workers in the polygynous stingless bee Melipona bicolor (Hymenoptera, Meliponini), Apidologie 38, 55–66.
Koedam D., Velthausz P.H., van de Krift T., Dohmen M.R., Sommeijer M.J. (1996) Morphology of reproductive and trophic eggs and their controlled release by workers in Trigona (Tetragonisca) angustula Illiger (Apidae, Meliponinae), Physiol. Entomol. 21, 289–296.
Kucharski R., Maleszka J., Foret S., Maleszka R. (2008) Nutritional control of reproductive status in honeybees via DNA methylation, Science 319, 1827–1830.
Kumaran R.I., Thakar R., Spector D.L. (2008) Chromatin dynamics and gene positioning, Cell 132, 929–934.
Lin H., Yue L., Spradling A.C. (1994) The Drosophila fusome, a germ line-specific organelle, contains membrane skeletal proteins and functions in cyst formation, Development 120, 947–956.
Machado M., Contel E.P.B., Kerr W.E. (1984) Proportion of males sons-of-the-queen and sons-of-the-workers in Plebeia droryana (Hymenoptera, Apidae) estimated from data of a MDH isozymic polymorphic system, Genetica 65, 193–198.
Makert G.R., Paxton R.J., Hartfelder K. (2006) Ovariole number — a predictor of differential success among worker subfamilies in queenless honeybee (Apis mellifera L.) colonies, Behav. Ecol. Sociobiol. 60, 815–825.
Martins G.F., Serrão J.E. (2004a) Changes in the reproductive tract of Melipona quadrifasciata anthidioides (Hymenoptera: Apidae, Meliponini) queen after mating, Sociobiology 44, 241–254.
Martins G.F., Serrão J.E. (2004b) A comparative study of the ovaries in some Brazilian bees (Hymenoptera; Apoidea), Pap. Av. Zool. 44, 45–53.
Melo G.A.R., Buschini M.L.T., Campos L.A.O. (2001) Ovarian activation in Melipona quadrifasciata queens triggered by mating plug stimulation (Hymenoptera, Apidae), Apidologie 32, 355–361.
Michener C.D. (1974) The Social Behavior of the Bees, Belknap Press of Harvard University Press, Cambridge.
Michener C.D. (2000) The Bees of the World, John Hopkins University Press, Baltimore.
Montague C.E., Oldroyd B.P. (1998) The evolution of worker sterility in hone bees: an investigation into a behavioral mutant phenotype casing failure in worker policing, Evolution 52, 1408–1415.
Nogueira-Neto P. (1997) Vida e Criação de Abelhas Indígenas sem Ferrão, Editora Nogueirapis, São Paulo.
Patel A., Fondrk M.K., Kaftanoglu O., Emore C., Hunt G., Frederick K., Amdam G.V. (2007) The making of a queen: TOR pathway is a key player in diphenic caste development, PLoS One 2(6), e509.
Paxton R.J., Bego L.R., Shah M.M., Mateus S. (2003) Low mating frequency of queens in the stingless bee Scaptotrigona postica and maternity of males, Behav. Ecol. Sociobiol. 53, 174–181.
Percipalle P., Visa N. (2006) Molecular functions of nuclear actin in transcription, J. Cell Biol. 172, 967–971.
Peters J.M., Queller D.C., Imperatriz-Fonseca V.L., Roubik D.W., Strassmann J.E. (1999) Mate number, kin selection and social conflicts in stingless bees and honeybees, Proc. R. Soc. Lond. Ser. B-Biol. Sci. 266, 379–384.
Rachinsky A., Hartfelder K. (1998) In vitro biosynthesis of juvenile hormone in larval honey bees: comparison of six media., In Vitro Cell Dev. Biol.-Animal 34, 646–648.
Ramamurty P.S. (1977) Unusual intertrophocytic position of follicle cells in the nurse chamber of the honeybee queen ovary (Apis mellifica), Apidologie 8, 205–216.
Rasmussen C., Camargo J.M.F. (2008) A molecular phylogeny and the evolution of nest architecture and behavior in Trigona s.s. (Hymenoptera: Apidae: Meliponini), Apidologie 39, 102–118.
Roubik D.W. (2006) Stingless bee nesting biology, Apidologie, 124–143.
Rübsam R., Büning J. (2001) F-actin is a component of the karyosome in neuropteran oocyte nuclei, Arthropod Struct. Dev. 30, 125–133.
Sakagami S.F. (1982) Stingless bees, in: Hermann H.R. (Ed.), Social Insects, Vol. 3. Academic Press, New York, pp. 361–423.
Schmidt Capella I.C., Hartfelder K. (1998) Juvenile hormone effect on DNA synthesis and apoptosis in caste-specific differentiation of the larval honey bee (Apis mellifera L.) ovary, J. Insect Physiol. 44, 385–391.
Silva P.G.F., Zucoloto F.S. (1990) A semiartificial diet for Scaptotrigona depilis, J. Apic. Res. 29, 233–235.
Snodgrass R.E. (1956) Anatomy of the Honey Bee, Cornell University Press, Ithaka, N.Y.
Tanaka E.D., Hartfelder K. (2004) The initial stages of oogenesis and their relation to differential fertility in the honey bee (Apis mellifera) castes, Arthropod Struct. Dev. 33, 431–442.
Toth E., Queller D.C., Dollin A., Strassmann J.E. (2004) Conflict over male parentage in stingless bees, Insect. Soc. 51, 1–11.
Velthuis H.H.W., Koedam D., Imperatriz-Fonseca V. (2005) The males of Melipona and other stingless bees, and their mothers, Apidologie 36, 169–185.
Warn R.M., Gutzeit H.O., Smith L., Warn A. (1985) Factin rings are associated with the Drosophila egg chamber canals, Exp. Cell Res. 157, 355–363.
Winston M.L. (1987) The Biology of the Honey Bee, Harvard University Press, Cambridge, Mass.
Zucchi R., Silva-Matos E.V., Nogueira-Ferreira F.H., Azevedo G.G. (1999) On the cell provisioning and oviposition process (POP) of the stingless — nomenclature reappraisal and evolutionary considerations (Hymenoptera, Apidae, Meliponinae), Sociobiology 34, 65–86.
Author information
Authors and Affiliations
Corresponding author
Additional information
Manuscript editor: Yves Le Conte
Rights and permissions
About this article
Cite this article
Tanaka, É.D., Santana, W.C. & Hartfelder, K. Ovariole structure and oogenesis in queens and workers of the stingless bee Melipona quadrifasciata (Hymenoptera: Apidae, Meliponini) kept under different social conditions. Apidologie 40, 163–177 (2009). https://doi.org/10.1051/apido/2008071
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1051/apido/2008071