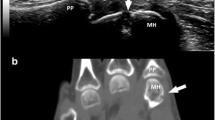
Abstract
The objective of this study is to assess the prevalence, localization, and severity of bone erosions on radiography (RX) and ultrasonography (US) according to ACPA status in patients with rheumatoid arthritis (RA). 78 patients with ACPA-positive (ACPA+) RA and 30 patients with ACPA-negative (ACPA−) RA fulfilling the ACR 1987 and/or ACR/EULAR 2010 criteria were consecutively included. On RX, a modified Sharp erosion score (SHSe) was evaluated by two blinded readers and one adjudicator for discordant cases (number of eroded joints ≤ three). On US, erosions were scored on six bilateral joints (MCP2, 3, 5; MTP2, 3, 5) with a four-point scale to calculate the total US score for erosions (USSe). The mean total SHSe and USSe were 3.7 and 4.4 times higher in the ACPA+ group than in the ACPA− group, respectively (P < 0.001). On both RX and US, the most discriminating joint between the two groups was MTP5, especially in cases with bilateral erosion. Based on multivariate analyses, ACPA + status was associated with erosive RA on RX according to the EULAR 2013 definition criteria [OR 4.4 (95% CI 1.2–16.4)], and on US according to the following two definitions: the presence of at least two eroded joint facets [OR 3.7 (95% CI 1.4–9.9)] or at least one grade 2 joint facet erosion [OR 9.0 (95% CI 2.8–28.4)]. Compared to ACPA− RA, ACPA + RA is associated independently with more severe erosive disease on RX and US. Both US and RX bilateral erosions in MTP5 joints are highly discriminant for ACPA + RA patients (97.8% in US and 100% in RX).


Similar content being viewed by others
References
Guillemin F, Saraux A, Guggenbuhl P, Roux CH, Fardellone P, Le Bihan E et al (2005) Prevalence of rheumatoid arthritis in France: 2001. Ann Rheum Dis 64:1427–1430
Alamanos Y, Voulgari PV, Drosos AA (2006) Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum 36:182–188
Vossenaar ER, Zendman AJW, van Venrooij WJ, Pruijn GJM (2003) PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays News Rev Mol Cell Dev Biol 25:1106–1118
Klareskog L, Rönnelid J, Lundberg K, Padyukov L, Alfredsson L (2008) Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol 26:651–675
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69:1580–1588
Jilani AA, Mackworth-Young CG (2015) The role of citrullinated protein antibodies in predicting erosive disease in rheumatoid arthritis: a systematic literature review and meta-analysis. Int J Rheumatol 2015:728610
Forslind K, Ahlmén M, Eberhardt K, Hafström I, Svensson B, BARFOT Study Group (2004) Prediction of radiological outcome in early rheumatoid arthritis in clinical practice: role of antibodies to citrullinated peptides (anti-CCP). Ann Rheum Dis 63:1090–1095
Baillet A, Gaujoux-Viala C, Mouterde G, Pham T, Tebib J, Saraux A et al (2011) Comparison of the efficacy of sonography, magnetic resonance imaging and conventional radiography for the detection of bone erosions in rheumatoid arthritis patients: a systematic review and meta-analysis. Rheumatology (Oxford) 50:1137–1147
Van der Heijde D, van Leeuwen M, van Riel P, van de Putte L (1995) Radiographic progression on radiographs of hands and feet during the first 3 years of rheumatoid arthritis measured according to Sharp’s method (van der Heijde modification). J Rheumatol 22:1792–1796
Van der Heijde D, Dankert T, Nieman F, Rau R, Boers M (1999) Reliability and sensitivity to change of a simplification of the Sharp/van der Heijde radiological assessment in rheumatoid arthritis. Rheumatology (Oxford) 38:941–947
Guillemin F, Billot L, Boini S, Gerard N, Ødegaard S, Kvien TK (2005) Reproducibility and sensitivity to change of 5 methods for scoring hand radiographic damage in patients with rheumatoid arthritis. J Rheumatol 32:778–786
Usón J, Fernández-Espartero C, Villaverde V, Condés E, Godo J, Martínez-Blasco MJ et al (2014) Symptomatic and asymptomatic interphalageal osteoarthritis: an ultrasonographic study. Reumatol Clin 10:278–282
Gutierrez M, Schmidt WA, Thiele RG, Keen HI, Kaeley GS, Naredo E et al (2015) International consensus for ultrasound lesions in gout: results of Delphi process and web-reliability exercise. Rheumatology (Oxford) 54:1797–1805
Weiner SM, Jurenz S, Uhl M, Lange-Nolde A, Warnatz K, Peter HH et al (2008) Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis: a comparison with radiography, MRI and scintigraphy. Clin Rheumatol 27:983–989
Wakefield RJ, Gibbon WW, Conaghan PG, O’Connor P, McGonagle D, Pease C et al (2000) The value of sonography in the detection of bone erosions in patients with rheumatoid arthritis: a comparison with conventional radiography. Arthritis Rheum 43:2762–2770
Bajaj S, Lopez-Ben R, Oster R, Alarcón GS (2006) Ultrasound detects rapid progression of erosive disease in early rheumatoid arthritis: a prospective longitudinal study. Skeletal Radiol 36:123–128
Van der Heijde D (2000) How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 27:261–263
Van der Heijde D, van der Helm-van Mil AHM, Aletaha D, Bingham CO, Burmester GR, Dougados M et al (2013) EULAR definition of erosive disease in light of the 2010 ACR/EULAR rheumatoid arthritis classification criteria. Ann Rheum Dis 72:479–481
Scheel AK (2006) Prospective 7 year follow up imaging study comparing radiography, ultrasonography, and magnetic resonance imaging in rheumatoid arthritis finger joints. Ann Rheum Dis 65:595–600
Szkudlarek M, Klarlund M, Narvestad E, Court-Payen M, Strandberg C, Jensen KE et al (2006) Ultrasonography of the metacarpophalangeal and proximal interphalangeal joints in rheumatoid arthritis: a comparison with magnetic resonance imaging, conventional radiography and clinical examination. Arthritis Res Ther 8:R52
Iagnocco A, Coari G (2000) Usefulness of high resolution US in the evaluation of effusion in osteoarthritic first carpometacarpal joint. Scand J Rheumatol 29:170–173
Zayat AS, Ellegaard K, Conaghan PG, Terslev L, Hensor EMA, Freeston JE et al (2015) The specificity of ultrasound-detected bone erosions for rheumatoid arthritis. Ann Rheum Dis 74:897–903
Roux C, Gandjbakhch F, Pierreisnard A, Couderc M, Lukas C, Masri R et al (2019) Ultrasonographic criteria for the diagnosis of erosive rheumatoid arthritis using osteoarthritic patients as controls compared to validated radiographic criteria. Jt Bone Spine 86(4):467–474
Roux C, Gandjbakhch F, Pierreisnard A, Couderc M, Lukas C, Masri R et al (2019) Optimization of ultrasonographic examination for the diagnosis of erosive rheumatoid arthritis in comparison to erosive hand osteoarthritis. Eur J Radiol 118:10–18
Gadeholt O, Hausotter K, Eberle H et al (2017) Erosion patterns in seropositive and seronegative rheumatoid arthritis: a joint-by-joint approach. ACR Meeting Abstracts. https://acrabstracts.org/abstract/erosion-patterns-in-seropositive-and-seronegative-rheumatoid-arthritis-a-joint-by-joint-approach. Accessed 22 Oct 2017
Szkudlarek M, Terslev L, Wakefield RJ, Backhaus M, Balint PV, Bruyn GAW et al (2015) Summary findings of a systematic literature review of the ultrasound assessment of bone erosions in rheumatoid arthritis. J Rheumatol 43:12–21
Tamas M-M, Filippucci E, Becciolini A, Gutierrez M, Di Geso L, Bonfiglioli K et al (2014) Bone erosions in rheumatoid arthritis: ultrasound findings in the early stage of the disease. Rheumatology 53:1100–1107
Ajeganova S, van Steenbergen HW, Verheul MK, Forslind K, Hafström I, Toes REM et al (2015) The association between anti-carbamylated protein (anti-CarP) antibodies and radiographic progression in early rheumatoid arthritis: a study exploring replication and the added value to ACPA and rheumatoid factor. Ann Rheum Dis 76:112–118
Schett G, Gravallese E (2012) Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 8:656–664
Nielen MMJ, van Schaardenburg D, Reesink HW, van de Stadt RJ, van der Horst-Bruinsma IE, de Koning MHMT et al (2004) Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 50:380–386
Van Schaardenburg D, Nielen MMJ, Lems WF, Twisk JWR, Reesink HW, van de Stadt RJ et al (2011) Bone metabolism is altered in preclinical rheumatoid arthritis. Ann Rheum Dis 70:1173–1174
Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E et al (2012) Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Investig 122:1791–1802
Kocijan R, Harre U, Schett G (2013) ACPA and Bone Loss in Rheumatoid Arthritis. Curr Rheumatol Rep 15:366
Guler H, Turhanoglu AD, Ozer B, Ozer C, Balci A (2008) The relationship between anti-cyclic citrullinated peptide and bone mineral density and radiographic damage in patients with rheumatoid arthritis. Scand J Rheumatol 37:337–342
Shidara K, Inoue E, Tanaka E, Hoshi D, Seto Y, Nakajima A et al (2011) Comparison of the second and third generation anti-cyclic citrullinated peptide antibody assays in the diagnosis of Japanese patients with rheumatoid arthritis. Rheumatol Int 31:617–622
Vos I, Van Mol C, Trouw LA, Mahler M, Bakker JA, Van Offel J et al (2017) Anti-citrullinated protein antibodies in the diagnosis of rheumatoid arthritis (RA): diagnostic performance of automated anti-CCP-2 and anti-CCP-3 antibodies assays. Clin Rheumatol 36:1487–1492
Zhang W-C, Wu H, Chen W-X (2014) Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide 2 antibody and anti-cyclic citrullinated peptide 3 antibody in rheumatoid arthritis. Clin Chem Lab Med CCLM 52:779–790
Barra L, Bykerk V, Pope JE, Haraoui BP, Hitchon CA, Thorne JC et al (2013) Anticitrullinated protein antibodies and rheumatoid factor fluctuate in early inflammatory arthritis and do not predict clinical outcomes. J Rheumatol 40:1259–1267
Kastbom A, Strandberg G, Lindroos A, Skogh T (2004) Anti-CCP antibody test predicts the disease course during 3 years in early rheumatoid arthritis (the Swedish TIRA project). Ann Rheum Dis 63:1085–1089
Author information
Authors and Affiliations
Contributions
JG and DL designed the study. AP, MC, and ICV performed the radiography assessment. DL and ICV performed the ultrasound assessment. MDC provided ACPA and RF values. JG and CR collected all of the data. EALL, ICU, TR, and EALB analyzed the data. JG, EALL, and DL aided in interpreting the results. JG wrote the paper with input from all authors. All co-authors approve the final version of the manuscript and take full responsibility for all its aspects.
Corresponding author
Ethics declarations
Conflict of interest
All of the authors declare that they have no conflict of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee [the ethical committee of Nancy approved the study in June 2017 (number: R2017-17)] and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grosse, J., Allado, E., Roux, C. et al. ACPA-positive versus ACPA-negative rheumatoid arthritis: two distinct erosive disease entities on radiography and ultrasonography. Rheumatol Int 40, 615–624 (2020). https://doi.org/10.1007/s00296-019-04492-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-019-04492-5