Abstract
Burnet moths (Zygaena spp.) are day-flying Lepidoptera considered indicative of species-rich grasslands. In the present study, our aim was to clarify whether clear-cuts are habitat, supporting habitat or matrix for three species of Zygaena. We did so by sampling these species with sex pheromones on 48 clear-cuts, varying in amount of host and nectar plants, in southern Sweden. To compare the efficiency of such sampling, we also conducted transect walks on these clearcuts. Overall, host-plants on clear-cuts best explained the abundance of Zygaena spp. recorded, better than nectar-plants or connectivity with nearby grasslands. These results indicate that clear-cuts with an abundance of host plants are used as a fully functional habitat, and not a supporting habitat in the sense of only providing nectar. There is no support in these results for considering clear-cuts as an inert matrix. With about half the work-effort, pheromone traps recorded 100 times more Zygaena spp. as transect walks. The poor correspondence between observations during transects walks and pheromone trap catches suggest Zygaena spp. being difficult to monitor by transect walks. In contrast to grasslands, clear-cuts are short-term in nature requiring repeated recolonization, indicating the importance of permanent grasslands. However, clear-cuts are important temporary insect habitats due to their great acreage, and suitable management can increase the time they remain a habitat.
Similar content being viewed by others
Introduction
Traditional agricultural landscapes are among Europe’s most species-rich areas. Substantial areas were historically subject to fodder production, mowing or grazing, and these semi-natural areas are today biodiversity hotspots (Poschlod and WallisDeVries 2002; Habel et al. 2013). However, the rapid change in agriculture has led to abandonment of low-productive areas and more intensified use of other areas (Ihse 1995; Eriksson et al. 2002). As a consequence, a severe decline in species-rich grassland and biodiversity of agricultural areas has been repeatedly reported in Europe (Kearns et al. 1998; Krebs et al. 1999; Bengtsson et al. 2000; Maes and Van Dyck 2001; Robinson and Sutherland 2002; Shrubb 2003; Foley et al. 2005).
A large proportion of European plants and insects prefer sun-exposed conditions and thrive in traditional agricultural landscapes (Ellenberg et al. 1991; Lindhe et al. 2005; Horák and Rébl 2013). An interesting debate is whether these sun-loving species assemblages were recruited from natural semi-open habitats created by large herbivores that are now extinct (e.g. Owen-Smith 1989; Vera 2000; van Vuure 2005; Feurdean et al. 2018; Ohwaki 2018), from grasslands originating from natural disturbances in the forests as floods, fires, storms or beavers (Ellenberg 1988; Pykälä 2000; Svenning 2002) or whether they have adapted to the features of the agricultural systems that evolved (Zopfi 1991, 1998; Lennartsson 1997). Whatever the origin, it has become increasingly clear that these species can sometimes be supported by other habitats that surrounds remaining grazed semi-natural grassland fragments (e.g. Berg et al. 2016; Bušek and Reif 2017; Lampinen et al. 2018; Bergman et al. 2018), often referred to as a matrix. The matrix is often neglected in butterfly studies; in a systematic review studying fragmented landscapes, 60% of the papers excluded the matrix (Sweaney et al. 2014). However, the matrix may contribute to survival and persistence of populations by providing resources (Dennis et al. 2006; Shreeve and Dennis 2011) like food (Dennis 2004; Brady et al. 2011), facilitating dispersal between patches (Jauker et al 2009; Kuefler et al 2010) or by decreasing negative edge effects (Lindenmayer et al. 2009; Ries and Sisk 2010). Hence, some matrix qualities might help mitigate the negative effects of fragmentation (Vaandermeer and Carvajal 2001; Jules and Shahani 2003; Lindenmayer and Fisher 2006; Frankling and Lindenmayer 2009).
Several studies of grassland butterflies have shown that a forest matrix decreases the negative effects of fragmentation of grasslands (Bergman et al. 2008, 2018; Öckinger et al. 2012; Villemey et al. 2015). Forest edges and clear-cuts in temperate climates may provide adult Lepidoptera with nectar sources and favourable microclimate for larval development (Dennis et al. 2004; Kuusaari et al. 2007; Van Halder et al. 2010; Ibbe et al. 2011; Jonason et al. 2014; Blixt et al. 2015; Korpela et al. 2015; Viljur and Teder 2016; Ohwaki et al. 2018a). Together with more permanent openings (Berg et al. 2013, 2016; Ohwaki et al. 2018b), a forest matrix may be important for conservation of grassland Lepidoptera by adding to the network of patches in metapopulation dynamics. One might even ask whether clear-cuts should be considered a “matrix” or a primary habitat for some presumed grassland species (Blixt et al. 2015).
To be able to successfully conserve species traditionally seen as agricultural grassland species there is a need to identify and characterize “alternative habitats”. Burnet moths (Zygaenidae) seem restricted to specific types of habitat (Ravenscroft and Young 1996; Crispin and Warrington 1997), and may be abundant if habitat is suitable (Bourn 1995; Naumann et al. 1999). The reduction of suitable habitats has affected the burnet moths severely (Wenzel et al. 2006) and five out of Sweden’s six species are red-listed (Ahrné et al. 2015). Furthermore, they are considered good indicators of species-rich semi-natural grasslands (Franzén and Ranius 2004). This makes them suitable to use when studying the importance of temporary habitats and land use history for conservation of burnet moths in Sweden. Recently, the identification and synthesis of sex pheromones has opened up a new frontier when it comes to investigations of rare insects (e.g. Musa et al. 2013; Andersson et al. 2014; Burman et al. 2016; Larsson 2016). Zygaena moths use sex pheromones and pheromones have been synthesised (Priesner et al. 1984; Subchev 2014; Oleander et al. 2015) for Zygaena osterodensis, Zygaena viciae and Zygaena filipendulae.
In the present study, we sampled these three species with pheromone traps in clear-cuts—an assumed alternative habitat—and used attributes of the clear-cuts to explain differences in occurrence and abundance. More specifically, we wanted to evaluate the relative importance of:
- 1
connectivity of clear-cuts to species-rich grasslands
- 2
nectar sources on clear-cuts
- 3
larval host plants on clear-cuts
We hoped to assess whether clear-cuts should be considered a habitat (mainly 2 and 3 important), a supportive habitat (mainly 1 and 2 important), or a matrix (mainly 1 important).
It is known that clear-cuts on land that was previously meadow 150 years ago, are richer in plants, grassland plants, and butterflies compared with areas of continued forest cover (Ibbe et al. 2011; Blixt et al. 2015; Jonason et al. 2014, 2016; Milberg et al. 2019). It is also known that the flora of clearcuts change rapidly in their first years (e.g. Schoonmaker and McKee 1988; Pykälä 2004). Therefore, the range of the two variables nectar and host plant abundances were maximized by choosing clear-cuts of different age (2–8 years) and land-use history (meadow or continuous forest).
Finally, as the clear-cuts had also been surveyed using conventional transect count methods in the same season as pheromone traps were used (Blixt et al. 2015), we wanted to compare the outcomes to evaluate the relative efficacy of the pheromone traps to transect counts.
Material and methods
Study species
Zygaena is a genus of moths in the family Zygaenidae. They are brightly coloured, day-flying and restricted to the western Palearctic. They prefer open and sunny biotopes and Zygaena larvae feed mainly on plants of the Fabaceae family, and the adults frequently use red and violet Dipsacaceae and Asteraceae flowers as nectar sources (Naumann et al. 1999; Sarin and Bergman 2010). Sweden has six species, of which five are on the red-list (Artdatabanken 2019a). Sex pheromones have been identified and synthesized for the species included in this study: Z. osterodensis, Z. viciae and Z. filipendulae. These species often co-occur, and their larvae feed mainly on one or a few species of Fabaceae (Söderström 2006).
Study area and selection of sites
The study was performed in southern Sweden in the province of Östergötland (N57°43′–58°15′; E15°00′–15°40′), in a landscape dominated by coniferous forest. The selection of clear-cuts is described in Blixt et al. (2015). Half of the clear-cuts had a management history as meadow and half as forest according to land use maps from the 1870s (Häradsekonomiska kartan, Jansson 1993; Runborg 1994). We selected clear-cuts between 1.5 and 7.0 ha in size (Table 1). We also selected clear-cuts according to the time since the cut (Table 1). Furthermore, we selected clear-cuts that were located at least 300 m from nearest seminatural grasslands (a distance longer than most reported average dispersal distances of Zygaena, but shorter than maximum dispersal distance, according to a review by Franzen and Nilsson 2007). Finally, the distance between two clear-cuts also needed to be at least 300 m. No other considerations were made regarding the surroundings of the clear-cuts, that was dominated by coniferous forest of different age. Butterfly data from the clear-cuts have previously been reported by Blixt et al. (2015) while vegetation data were reported by Jonason et al. (2014).
Sex pheromone lures
Lures were prepared from grey rubber septa (PheroNet, Sweden) according to the methods of Burman et al. (2016), using compounds obtained from PheroBank, The Netherlands. The pheromone used for Z. filipendulae was a blend of Z7-12:Ac, Z9-14:Ac, Z5-12:Ac in the proportion of 100/10/3 µg per septa and for Z. viciae the same substances in the proportion of 100/10/10 µg, as published by Priesner et al. (1984). The pheromone blend used for Z. osterodensis was Z7-12:Ac, Z9-14:Ac in the proportion of 100/100 µg per septa, based on unpublished data by Ernst Priesner and Nils Ryrholm.
Sampling of burnet moths
Three sticky traps (transparent plastic delta traps; Csalomon, Budapest, Hungary) with one pheromone lure each were distributed at each clear-cut and left for one week. Traps were hung from a shrub, small tree or logging debris, at about breast height. The placement of traps on a clear-cut aimed at (i) selecting trap locations that were representative for that particular clear-cut, and (ii) the three trap locations being as similar as possible. Traps for Z. osterodensis were put up in the first week of July 2013, while the other traps were put up two weeks later, reflecting the flight period of the species.
Transect walks were conducted on three occasions during 2013, following standard procedures for this methodology (full details given in Blixt et al. 2015). Only two of the transect walks (June 17 to July 11; and July 17 to Aug. 3) occurred during the flight period of Zygaena species sampled. Walks were conducted between 09:00 and 17:00 (UTC + 2) at temperatures above 17 °C and under predominantly sunny conditions, with winds of up to level 4 on the Beaufort scale. Transects were walked at a constant pace of 50 m/min. Transect lines were 25 m apart, and all specimens within an area of 5 m in front, 5 m to each side and 5 m up in the air were identified to species level. In this way 40% of each clear-cut was covered.
Connectivity
Species-rich grassland in the study area was identified using the TUVA database, administered by the Swedish Board of Agriculture. The database is the result of field inventories searching species-rich grasslands. Connectivity (Hanski 1994) for a clear-cut i, to nearby species-rich grasslands, was calculated using Ci = ∑jexp(− dij/α)Aj, where the areas of neighboring grasslands (Aj) are summed after scaling with the distance from i to j (dij) and a scaling factor (α = 1.4). The latter corresponds to “average dispersal distance”, and 1.4 was chosen based on Franzen and Nilsson (2007) who report such distances for Z. viciae being 1.1 and 1.8 km (two different years). Connectivity estimates were numerically skewed and therefore square-root transformed before analyses.
Vegetation sampling
In 2013, plant presence was recorded within 100 circular sample plots (radius 1 m) placed evenly throughout each clear-cut along transects spaced 25 m apart. The number of sample plots in which a species was present was taken as a measure of its frequency. The vegetation data has been presented elsewhere (Jonason et al. 2014, 2016).
Nectar sources
Adult Zygaena use several plant species for nectar, but some species are of particular importance. Based on literature (Naumann et al. 1999; Sarin and Bergman 2010) and the occurrence of species in our vegetation sample, we calculated an index that was the sum of frequencies of the following species on a clear-cut: Knautia arvensis, Cirsium spp., Rubus fruticosus coll. and Trifolium spp. As Knautia arvensis seems to be very important (Lack 1982; Holbeck et al. 2000; Sarin and Bergman 2010; unpublished data), we arbitrarily doubled its frequency when calculating the sum. As this nectar index was numerically skewed, it was square-root transformed before analyses.
Host plants
Although larvae of Zygaena seem to use only, or mainly, species of Fabaceae, there also seem to be different preferences among Zygaena species (e.g. Söderström 2006) within this plant family. For these reasons we calculated one host plant index per species, all being the sum of all frequencies on a clear-cut of the plant species that Artdatabanken lists as hostplants (http://artfakta.artdatabanken.se/taxon/1000572):
- 1
Z. osterodensis: Vicia cracca, Vicia sylvatica and Lathyrus pratensis
- 2
Z. viciae: Lotus corniculatus, Lathyrus pratensis, Vicia spp. and Trifolium spp.
- 3
Z. filipendulae: Lotus corniculatus
These host plant indices were all numerically skewed, and was therefore square-root transformed before analyses.
Statistical analysis
Species-wise generalized linear models (GLM with Negative bionomial distribution and log-link) were used to assess the relative importance of three clear-cut attributes (explanatory variables) for the number of Zygenae trapped: connectivity, nectar and host plants. As the three variables were correlated (Table 2), one analysis was conducted per explanatory variable and species. Analyses were conducted with the software Statistica 13 (TIBCO Software Inc.).
Results
In total, 1075 Zygaena individuals were caught, of which 45.9%, 33.4%. 20.7% were of Z. osterodensis, Z. viciae and Z. filipendulae, respectively. Zygaena specimens were recorded on all clear-cuts, from a minimum of 2 to a maximum of 123 (divided among three traps per clear-cut). Zygaena osterodensis, Z. viciae and Z. filipendulae were not recorded on 7, 5 and 7 clearcuts, respectively.
Zygaena osterodensis traps caught only the intended species, which is not surprising as it has an earlier flight period than the other species (n.b. its traps were put up two weeks earlier than the other traps). For the traps that were up simultaneously, targeting species with similar flight period, 26.5% of Z. viciae were caught in a trap targeting Z. filipendulae, and 30.5% of the Z. filipendulae were recorded in a trap targeting Z. viciae. Low specificity was expected due to the similarity of the pheromone blends.
Most Zygaena were caught by pheromone trapping in the clear-cuts that had previously been meadows (72.5%), which were also richer in nectar and host plants, but did not differ in connectivity from clear-cuts with a history as forest (Fig. 1).
Abundance of Zygaena
Overall, host plant index was the most important explanatory variable for the abundance of two of the three species while there were substantial differences among the species (Table 3). The abundance of Z. osterodensis could be explained by host plants (highest Wald, significant) and nectar plants. For Z. viciae, all three variables were highly significant, with nectar plants being the best model, followed by host plant (Table 3). Only host plants could explain Z. filipendulae abundance (Table 3).
Comparing transect walk data and pheromone trap catches
During transect walks, in total 10 specimens of two species of Zygaena, were recorded compared with 1075 specimens of three species in pheromone traps. Zygaena viciae was not recorded during transect walks but made up 33% of the trap catches and was recorded at 43 of the 48 clear-cuts. Only a single Z. filipendulae was seen in transects while contributing to 21% of trap catches being recorded on 41 of the clear-cuts.
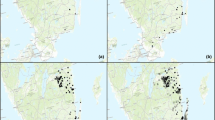
In seven clear-cuts, a total of nine specimens of Z. osterodensis were seen during walks. In contrast, this species made up 46% of the trap catch, being recorded in 41 clear-cuts. The numerical correspondence between observations and trap catches was low with, e.g., observations on 2 of the 7 clear-cuts lacking trap catches (Fig. 2).
Discussion
Clear-cuts: habitat, supportive habitat, or matrix?
The perfect answer to our key question would emerge from studies of population dynamics. Such studies are unlikely to be feasible, however, given the mobility of the species involved, and the need to record fecundity and juvenile survival on different host plants and areas, etc. An alternative approach for these insects is to explore the relative importance of occurrence in the field, of (i) distance to presumed main habitat, (ii) nectar plants and (iii) host plants. The abundance of host plants was the strongest explanatory factor for the abundance of two species and the close second for the third, with consistently larger explanatory power than connectivity. Connectivity had only one case of significance (Z. viciae). These results suggest that clear-cuts are used as a fully functional habitat, and not a supporting habitat in the sense of only providing nectar. There is no support in these results for considering clear-cuts as an inert matrix, in which case only connectivity should have explanatory power. If clear-cuts are habitat, a grassland-based connectivity estimate is not meaningful for Zygaena, and a relevant estimate should include also clear-cuts.
Of the three species, Z. osterodensis was least affected by connectivity (lowest Wald values). This confirm previous reports stating that this species frequently use glades, forest edges and forest roads. Although a species occurring also in semi-natural grasslands in agricultural landscapes, our results indicate that it is more of a forest species than the other two species (Artdatabanken 2019b).
Even if Zygaena spp. use clear-cuts as a habitat, clear-cuts themselves are temporary with a lifespan of about a decade (Jonason et al. 2016) after which a tree canopy is forming and shading increases. This overgrowth makes the habitat deteriorate as nectar sources and host plants decreases. Hence, in the longer time perspective, Zygaena spp. will have to repeatedly colonize new clear-cuts from nearby habitats, in a manner similar to that documented for Mellicta athalia and its occurrence in coppice woodland, a habitat that is open and suitable for a limited number of years forcing constant re-colonisation (Warren 1987a, b). Nearby habitats might constitute grasslands, road verges and powerlines—that are permanent habitats—and clear-cuts that are temporary (cf Wahlberg et al. 2002). According to classical metapopulation understanding, there would be a lower limit to the connectivity or number of patches, below which long-term survival is unlikely (Hanski 1998). In fact, this does seem to be the case, as some Zygaena species are absent from forest-dominated areas in southern Sweden (Sarin and Bergman 2010), suggesting that permanent habitats like grasslands become important on the larger landscape scale (Bergman et al. in prep). Wahlberg et al. (2002) made a similar conclusion when studying the population dynamics of the butterfly Euphydryas aurinia occurring in a landscape with a mix of clear-cuts and permanent grasslands. Their simulations indicated that clear-cuts on their own were not enough for long-term survival of the species; permanent grasslands were needed for continued presence in the landscape.
Previous studies have shown that historical management influenced butterfly and plant species in clear-cuts in the study areas. It is difficult to isolate the causal chain as connectivity, history, and plant species composition are interrelated. Still, Zygaena spp. do use clear-cuts as habitat, and rely on certain plants (nectar, hostplant) that are more likely to be encountered on clear-cuts on land affected by previous agricultural practices. To sum up, due to their prevalence in many landscapes, clear-cuts are important habitats likely affecting the geographic distribution of many Zygaenae. Furthermore, landuse history influence the vegetation on clear-cuts (Jonason et al. 2014, 2016) and can thereby locally boost Zygaena populations in a way similar to that documented for butterflies (Ibbe et al. 2011; Berg et al. 2011; Blixt et al. 2015; Viljur and Teder 2016; Ohwaki et al. 2018a).
Pheromones and monitoring
Pheromone traps recorded 100 times more Zygaena specimens than did the transect walks. The time invested in fieldwork depends on logistics and, in the case of transect walks, weather. The current pheromone trapping needed four visits to a site (to set up and take down traps targeting the early-flying Z. osterodensis, then traps for the other two species; using slightly prolonged periods, the pheromone monitoring could have been done with three visits). In contrast, the transect walks needed only three visits. On the other hand, transect walks are more time-consuming and weather-dependent. Our two field workers sampled on average 6 clear-cuts per day using transect walks (SD 3.5; range 1–15), needing in total 48 man-days in the field. Assuming a person can visit 10 clear-cuts in a day in our study area, it would take less than 20 man-days to manage the pheromone trapping. Hence, with less than half the work effort, pheromone traps caught 100 times more Zygaena than were recorded in transect walks in the present study, which points to the potential importance of pheromone trapping for monitoring. It is worth pointing out that we used transect walk methodology developed for day-flying butterflies, and methods specifically developed for Zygaena would probably result in more reliable data.
Given the efficacy of traps, it is important to adjust the catching effort not to negatively affect populations. For example, refining the size and number of the traps, and limiting the area of the sticky surface in a trap are ways to minimize potential negative effects of using pheromones for monitoring Zygaena. Also, the days in the field can be adjusted during fieldwork and thereby fine-tuned to achieve an appropriate catch. Finally, it is worth pointing out the prospect of using live traps in monitoring (e.g. Andersson et al. 2014; Oleander et al. 2019), a hitherto unexplored option for monitoring of Zygaena spp. A drawback with live traps, however, is the need to empty them frequently (preferably daily for Zygaena), which limits the scale of sampling. But if monitoring is conducted as ‘citizen science’, there would be less constraints on labour. An interesting alternative to both sticky and live traps is if game cameras can be fine-tuned for burnet moths and baited with pheromones.
There was very poor correspondence between observations during transects walks and pheromone trap catches. Theoretically, this could be ascribed to transect walks under-reporting and/or pheromone traps over-reporting. There is some indication that Zygaena turn up during transect walks to less extent than butterflies, as the former seem to preferentially fly in the afternoon (Franzén and Nilsson 2007; Wikström et al. 2009), suggesting they are sensitive to low temperatures prevailing earlier in the day. There might be a risk that pheromone traps, if highly efficient or targeting a very mobile species, attract specimens from outside of the intended sampling area. Zygaena spp. seems to be relatively mobile, with mark-recapture studies recording dispersals up to 5600 m, and with 8% of recaptures in a different habitat patch (Franzen and Nilsson 2007, 2012). Nevertheless, the evidence suggests that direct odour-guided attraction to pheromone sources only occurs within tens to a few hundred metres (e.g. Schlyter 1992) which seems well-suited to the size of our sampled areas (clear-cuts of a few ha). Another line of argument, for pheromone trapping not being overly efficient, is that the traps failed to catch Z. filipendulae on two clear-cuts where this species had been seen during transect walks. Finally, in two catch-and-release trials involving Z. filiendulae in the UK, 58 individuals were recaptured after releasing from increasing distances from a centroid pheromone trap, of which only 2 returned from 100 and 120 m of the release point respectively (Burman et al. unpublished).
Forestry management implications and conservation
The current study adds to the growing evidence that clear-cuts are important, temporary Lepidoptera habitats, including for many red listed species. It has previously been proposed (Ibbe et al. 2011; Jonason et al. 2014, 2016; Blixt et al. 2015; Milberg et al. 2019) that such habitats can be boosted, at least on land with a grassland legacy, by planting of deciduous trees (more light to ground compared with conifers like Picea abies), increasing planting distances (that would delay the effect of tree canopy closure), or leaving some areas for free development (i.e. creating future glades in plantations). A more extreme measure would be to introduce forest grazing, a currently subsidized practice (Westin and Lennartsson 2018).
An open question remains regarding clear-cuts: if the presence of Zygaena hostplants on forested land does reflect previous grassland land use (Jonason et al. 2014, 2016; Milberg et al. 2019), or previous open, grazed forests (a practice that ceased during the early 1900s), then how much of this botanical legacy will remain after an additional forestry cycle? Considering that the next forestry cycle will be much denser than the previous one (Hedwall and Brunet 2016; Bergstedt et al. 2017; Pettersson et al. 2019), the legacy seems unlikely to prevail long-term, unless some measures are taken (see above).
Conclusions
This study suggests that clear-cuts in boreal forests are a fully functional habitat for three species of Zygaena studied. Given how prevalent clear-cuts are, they constitute a very significant habitat, albeit temporary, that likely affect the geographic distribution of many Zygaenae. Furthermore, clear-cuts on land that were used as meadow 150 years ago were particularly rich in Zygaena. Using sex pheromones to sample Zygaena proved very efficient compared with transect walks, and especially if live or camera traps can be developed, seems promising for monitoring.
References
Ahrné K, Bengtsson B, Björklund JO, Cederberg B, Eliasson C, Hydén N, Jonasson J, Lindeborg M, Ohlsson A, Palmqvist G, Ryrholm N (2015) Rödlistade fjärilar, Red listed Butterflies and Moths (Lepidoptera). In: Gärdenfors U (ed) Rödlistade arter i Sverige 2015. ArtDatabanken SLU, Uppsala, pp 98–112
Andersson K, Bergman K-O, Andersson F, Hedenström E, Jansson N, Burman J, Winde I, Larsson MC, Milberg P (2014) High-accuracy sampling of saproxylic diversity indicators at regional scales with pheromones: the case of Elater ferrugineus (Coleoptera, Elateridae). Biol Conserv 171:156–166
Artdatabanken (2019a) Zygaena. http://artfakta.artdatabanken.se/taxon/1000572. Accessed 23 Sep 2019
Artdatabanken (2019b) Zygaena osterodensis. http://artfakta.artdatabanken.se/taxon/102020. Accessed 23 Sep 2019
Bengtsson J, Nilsson SG, Franc A, Menozzi P (2000) Biodiversity, disturbances, ecosystem function and management of European forests. For Ecol Manag 132:39–50
Berg Å, Ahrné K, Öckinger E, Svensson R, Söderström B (2011) Butterfly distribution and abundance is affected by variation in the Swedish forest-farmland landscape. Biol Conserv 144:2819–2831
Berg Å, Ahrné K, Öckinger E, Svensson R, Wissman J (2013) Butterflies in semi-natural pastures and power-line corridors: effects of flower richness, management, and structural vegetation characteristics. Insect Conserv Divers 6:639–657
Berg Å, Bergman KO, Wissman J, Żmihorski M, Öckinger E (2016) Power-line corridors as source habitat for butterflies in forest landscapes. Biol Conserv 201:320–326
Bergman K-O, Ask L, Askling J, Ignell H, Wahlman H, Milberg P (2008) Importance of boreal grasslands in Sweden for butterfly diversity and effects of local and landscape habitat factors. Biodivers Conserv 17:139–153
Bergman K-O, Dániel Ferreira J, Milberg P, Öckinger E, Westerberg L (2018) Landscape mediated patterns of butterfly occurrence in semi-natural grasslands. Landsc Ecol 33:2189–2204
Bergstedt J, Axelsson A-L, Karlsson J, Lönander J, Törnqvist L, Milberg P (2017) Förändringar i Eklandskapet 1927 till 2013: i den första Riksskogstaxeringens fotspår. Sven Bot Tidskr 111:331–343
Blixt T, Bergman K-O, Milberg P, Westerberg L, Jonason D (2015) Clear-cuts in production forests are not matrix, but neo-habitats for butterflies. Acta Oecol 69:71–77
Bourn NAD (1995) The ecology, conservation and population genetics of three species of Zygaenid moths, Zygaena lonicerae, Zygaena purpuralis and Zygaena filipendulae in North west Scotland. PhD thesis, University of Aberdeen
Brady M, McAlpine C, Possingham H, Miller C, Baxter G (2011) Matrix is important for mammals in landscapes with small amounts of native forest habitat. Landsc Ecol 26:617–628
Burman J, Westerberg L, Ostrow S, Ryrholm N, Bergman K-O, Winde I, Nyabuga FN, Larsson MC, Milberg P (2016) Revealing hidden species distribution with pheromones: the case of Synanthedon vespiformis (Lepidoptera: Sesiidae) in Sweden. J Insect Conserv 20:11–21
Bušek O, Reif J (2017) The potential of military training areas for bird conservation in a central European landscape. Acta Oecol 84:34–40
Crispin MJ, Warrington S (1997) Aspects of the population ecology of Zygaena filipendulae (Linnaeus) (Lepidoptera: Zygaenidae). Entomol Gazette 48:97–105
Dennis RLH (2004) Butterfly habitats, broad-scale biotope affiliations, and structural exploitation of vegetation at finer scales: the matrix revisited. Ecol Entomol 29:744–752
Dennis RLH, Hodgson JG, Grenyer R, Shreeve TG, Roy DB (2004) Host plants and butterfly biology. Do host-plant strategies drive butterfly biology? Ecol Entomol 29:12–26
Dennis RLH, Shreeve TG, Van Dyck H (2006) Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers Conserv 15:1943–1966
Ellenberg HH (1988) Vegetation ecology of central Europe. Cambridge University Press, Cambridge
Ellenberg HH, Weber HE, Dull R, Wirth V, Werner W, Paulissen D (1991) Zeigerwerte von Pflanzen in Mitteleuropa. Scr Geobot 18:248
Eriksson O, Cousins SA, Bruun HH (2002) Land-use history and fragmentation of traditionally managed grasslands in Scandinavia. J Veg Sci 13:743–748
Feurdean A, Ruprecht E, Molnár Z, Hutchinson SM, Hickler T (2018) Biodiversity-rich European grasslands: ancient, forgotten ecosystems. Biol Conserv 228:224–232
Foley JA, DeFries R, Asner GP, Barford C, Bonan G, Carpenter SR, Chapin FS, Coe MT, Daily GC, Snyder PK (2005) Global consequences of land use. Science 309:570–574
Frankling JF, Lindenmayer DB (2009) Importance of matrix habitats in maintaining biological diversity. Proc Natl Acad Sci 106:349–350
Franzén M, Nilsson SG (2007) What is the required minimum landscape size for dispersal studies? J Anim Ecol 76:1224–1230
Franzén M, Nilsson SG (2012) Climate-dependent dispersal rates in metapopulations of burnet moths. J Insect Conserv 16:941–947
Franzén M, Ranius T (2004) Habitat associations and occupancy patterns of burnet moths (Zygaenidae) in semi-natural pastures in Sweden. Entomol Fennica 15:91–101
Habel JC, Dengler J, Janišová M, Török P, Wellstein C, Wiezik M (2013) European grassland ecosystems: threatened hotspots of biodiversity. Biodivers Conserv 22:2131–2138
Hanski I (1994) A practical model of metapopulation dynamics. J Anim Ecol 63:151–162
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49
Hedwall PO, Brunet J (2016) Trait variations of ground flora species disentangle the effects of global change and altered land-use in Swedish forests during 20 years. Glob Change Biol 22:4038–4047
Holbeck HB, Clausen HD, Reddersen J (2000) Selection of nectar sources by butterflies and burnets in organic field boundary habitats (Papilionoidea, Hesperioidea and Zygaenidae). Entomol Medd 68:47–59 (In Danish)
Horák J, Rébl K (2013) The species richness of click beetles in ancient pasture woodland benefits from a high level of sun exposure. J Insect Conserv 17:307–318
Ibbe M, Milberg P, Tunér A, Bergman K-O (2011) History matters: impact of historical landuse on butterfly biodiversity in clear-cuts in boreal landscape. For Ecol Manag 261:1885–1891
Ihse M (1995) Swedish agricultural landscapes: pattern and changes during the last 50 issueds, studied by aerial photos. Landsc Urban Plan 31:21–37
Jansson U (1993) Ekonomiska kartor 1800–1934. Riksantikvarieämbetet. Almqvist & Wiksell Tryckeri, Uppsala
Jauker F, Diekötter T, Schwarzbach F, Wolters V (2009) Pollinator dispersal in an agricultural matrix: opposing responses of wild bees and hoverflies to landscape structure and distance from main habitat. Landsc Ecol 24:547–555
Jonason D, Ibbe M, Milberg P, Tunér A, Westerberg L, Bergman K-O (2014) Vegetation in clear-cuts depends on previous land use: a century-old grassland legacy. Ecol Evol 4:4287–4295
Jonason D, Bergman K-O, Westerberg L, Milberg P (2016) Land-use history exerts long-term effects on the flora in clear-cuts. Appl Veg Sci 19:634–643
Jules ES, Shahani P (2003) A broader ecological context to habitat fragmentation: why matrix habitat is more important than we thought. J Veg Sci 14(3):459–464
Kearns CA, Inouye DW, Waser NM (1998) Endangered mutualism: the conservation of plant–pollinator interactions. Ann Rev Ecol Syst 29:83–112
Korpela E-L, Hyvönen T, Kuusaari M (2015) Logging in boreal field-forest ecotones promotes flower-visiting insect diversity and modifies insect community composition. Insect Conserv Divers 8:152–162
Krebs JR, Wilson JD, Bradbury RB, Siriwardena GM (1999) The second silent spring? Nature 400:611–612
Kuefler D, Hudgens B, Haddad NM, Morris WF, Thurgate N (2010) The conflict role of matrix habitats as conduits and barriers for dispersal. Ecology 91:994–950
Kuusaari M, Heliölä J, Luoto M, Pöyru J (2007) Determinants of local species richness of diurnal Lepidoptera in boreal agricultural landscapes. Agr Ecosyst Environ 122:366–376
Lack AJ (1982) The ecology of flowers of chalk grassland and their insect pollinators. J Ecol 70:773–790
Lampinen J, Heikkinen RK, Manninen P, Ryttäri T, Kuussaari M (2018) Importance of local habitat conditions and past and present habitat connectivity for the species richness of grassland plants and butterflies in power line clearings. Biodivers Conserv 27:217–233
Larsson MC (2016) Pheromones and other semiochemicals for monitoring rare and endangered species. J Chem Ecol 42:853–868
Lennartsson T (1997) Seasonal differentiation—a conservative reproductive barrier in two grassland Gentianella (Gentianaceae) species. Plant Syst Evol 208:45–69
Lindenmayer DB, Fischer J (2006) Habitat fragmentation and landscape change: an ecological and conservation synthesis. CSIRO Publishing, Canberra
Lindenmayer DB, Wood JT, Cunningham RB, Crane M, Macgregor C, Michael D, Montauge-Drake R (2009) Experimental evidence of the effects of a changed matrix on conserving biodiversity within patches of native forest in an industrial plantation landscape. Landsc Ecol 24:1091–1103
Lindhe A, Lindelöw Å, Åsenblad N (2005) Saproxylic beetles in standing dead wood density in relation to substrate sun-exposure and diameter. Biodivers Conserv 14:3033–3053
Maes D, Van Dyck H (2001) Butterfly diversity loss in Flanders (north Belgium): Europe’s worst case scenario? Biol Conserv 99:263–276
Milberg P, Bergman K-O, Jonason D, Karlsson J, Westerberg L (2019) Land-use history influence the vegetation in coniferous production forests in southern Sweden. For Ecol Manag 440:23–30
Musa N, Andersson K, Burman J, Andersson F, Hedenström E, Jansson N, Paltto H, Westerberg L, Winde I, Larsson MC, Bergman K-O, Milberg P (2013) Using sex pheromone and a multi-scale approach to predict the distribution of a rare saproxylic beetle. PLoS ONE 8(6):e66149
Naumann CM, Tarmann GM, Tremewan WG (1999) Western Palaearctic Zygaenidae. Apollo Books, Stenstrup
Öckinger E, Bergman K-O, Franzén M, Kadlec T, Krauss J, Kuussaari M, Pöyry J, Smith HG, Steffan-Dewenter I, Bommarco R (2012) The landscape matrix modifies the effect of habitat fragmentation in grassland butterflies. Landsc Ecol 27:121–131
Ohwaki A (2018) How should we view temperate semi-natural grasslands? Insights from butterflies in Japan. Glob Ecol Conserv 16:e00482
Ohwaki A, Koyanagi TF, Maeda S (2018) Evaluating forest clear-cuts as alternative grassland habitats for plants and butterflies. For Ecol Manag 430:337–345
Ohwaki A, Hayami SI, Kitahara M, Yasuda T (2018) The role of linear mown firebreaks in conserving butterfly diversity: Effects of adjacent vegetation and management. Entomol Sci 21:112–123
Oleander A, Thackery D, Burman J (2015) The effect of exposure to synthetic pheromone lures on male Zygaena filipendulae mating behaviour: implications for monitoring species of conservation interest. J Insect Conserv 19:539–546
Oleander A, Bray DP, Hall DR, Burman JPJ (2019) Identification of female sex pheromone for monitoring the Barred Tooth Striped Moth, Trichopteryx polycommata, a priority conservation species. J Chem Ecol 45:649–656
Owen-Smith N (1989) Megafaunal extinctions: the conservation message from 11,000 years BP. Conserv Biol 3:405–412
Petersson L, Milberg P, Bergstedt J, Dahlgren J, Felton A, Götmark F, Salk C, Löf M (2019) Changed land use and deer overabundance cause natural oak regeneration failure: six decades of landscape scale evidence. For Ecol Manag 444:299–307
Poschlod P, WallisDeVries MF (2002) The historical and socioeconomic perspective of calcareous grasslands: lessons from the distant and recent past. Biol Conserv 104:361–376
Priesner E, Naumann CM, Stertenbrink J (1984) Specificity of synthetic sex-attractants in Zygaena moths. Z Naturforsch C 39:841–844
Pykälä J (2000) Mitigating human effects on European biodiversity through traditional animal husbandry. Conserv Biol 14:705–712
Pykälä J (2004) Immediate increase in plant species richness after clear-cutting of boreal herb-rich forests. Appl Veg Sci 7:29–34
Ravenscroft NO, Young MR (1996) Habitat specificity, restricted range and metapopulation persistence of the slender scotch burnet moth Zygaena loti in western Scotland. J Appl Ecol 33:993–1000
Ries L, Sisk TD (2010) What is an edge species? The implications of sensitivity to habitat edges. Oikos 119:1636–1642
Robinson RA, Sutherland WJ (2002) Post-war changes in arable farming and biodiversity in Great Britain. J Appl Ecol 39:157–176
Runborg S (1994) Historiska kartor: underlag för natur- och kulturmiljövård i skogen. Skogsstyrelsen Rapport 1994:5, 42 p
Sarin C, Bergman K-O (2010) Habitat utilisation of burnet moths (Zygaena spp.) in southern Sweden: a multi-scale and multi-stage perspective. Insect Conserv Divers 3:180–193
Schlyter F (1992) Sampling range, attraction range, and effective attraction radius: Estimates of trap efficiency and communication distance in coleopteran pheromone and host attractant systems. J Appl Entomol 114:439–454
Schoonmaker P, McKee A (1988) Species composition and diversity during secondary succession of coniferous forests in the western Cascade Mountains of Oregon. For Sci 34:960–979
Shreeve TG, Dennis RLH (2011) Landscape scale conservation: resources, behaviour, the matrix and opportunities. J Insect Conserv 15:179–188
Shrubb M (2003) Birds, scythes and combines: a history of birds and agricultural change. Cambridge University Press, Cambridge
Söderström B (2006) Svenska fjärilar: en fälthandbok. Bonnier, Stockholm
Subchev M (2014) Sex pheromone communication in the family Zygaenidae (Insecta: Lepidoptera): a review. Acta Zool Bulg 66:147–157
Svenning JC (2002) A review of natural vegetation openness in north-western Europe. Biol Conserv 104:133–148
Sweaney N, Lindenmayer DB, Driscoll DA (2014) Is the matrix important to butterflies in fragmented landscapes? J Insect Conserv 18:283–294
Vandermeer J, Carvajal R (2001) Metapopulation dynamics and the quality of the matrix. Am Nat 158:211–220
Van Halder I, Barbaro L, Jactel H (2010) Conserving butterflies in fragmented plantation forests: are edge and interior habitats equally important? J Insect Conserv 15:591–601
Van Vuure C (2005) Retracing the aurochs: history, morphology and ecology of an extinct wild ox. Pensoft Pub
Vera FWM (2000) Grazing ecology and forest history. CABI publishing, New York
Viljur M-L, Teder T (2016) Butterflies take advantage of contemporary forestry: clear-cuts as temporary grasslands. For Ecol Manage 376:118–125
Villemey A, van Halder I, Ouin A, Barbaro L, Chenot J, Tessier P et al (2015) Mosaic of grasslands and woodlands is more effective than habitat connectivity to conserve butterflies in French farmland. Biol Conserv 191:206–215
Wahlberg N, Klemetti T, Hanski I (2002) Dynamic populations in a dynamic landscape: the metapopulation structure of the marsh fritillary butterfly. Ecography 25:224–232
Warren MS (1987a) The ecology and conservation of the heath fritillary butterfly, Mellicta athalia. II. Adult population structure and mobility. J Appl Ecol 24:483–498
Warren MS (1987b) The ecology and conservation of the heath fritillary butterfly, Mellicta athalia. III. Population dynamics and the effect of habitat management. J Appl Ecol 24:499–513
Wenzel M, Schmitt T, Weitsel M, Seitz A (2006) The severe decline of butterflies on western German calcareous grasslands during the last 30 issueds: a conservation problem. Biol Conserv 128:542–552
Westin A, Lennartsson T (2018) Skogsbetesmarker i Sverige: historia, ekologi, natur- och kulturmiljövård. SLU, Uppsala, Centrum för bioloigisk mångfald
Wikström L, Milberg P, Bergman K-O (2009) Monitoring of butterflies in semi-natural grasslands: diurnal variation and weather effects. J Insect Conserv 13:203–211
Zopfi HJ (1991) Aestival and autumnal vicariads of Gentianella (Gentianaceae): a myth? Plant Syst Evol 174:139–158
Zopfi HJ (1998) Life-history variation among populations of Euphrasia rostkoviana Hayne (Scrophulariaceae) in relation to grassland management. Biol J Lin Soc 64:179–205
Acknowledgements
Open access funding provided by Linköping University. Financial support was provided by the Swedish Forest Society (to KOB) and WWF Sweden (Grant: Insight: SWE 0163; Local: 500 131). We thank Boxholms Skogar AB and private forest owners for allowing us to perform fieldwork on their land, and Torbjörn Blixt and Staffan Carlsson for assistance in the field.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts of interest in this study.
Research involving animals
This study involves trapping insects for which no permit is needed in Sweden.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bergman, KO., Burman, J., Jonason, D. et al. Clear-cuts are temporary habitats, not matrix, for endangered grassland burnet moths (Zygaena spp.). J Insect Conserv 24, 269–277 (2020). https://doi.org/10.1007/s10841-019-00193-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-019-00193-3