Abstract
Development of childhood asthma is complex with a strong interaction of genetic, epigenetic, and environmental factors. Ultimately, it is critical how the immune system of a child responds to these influences and whether effective strategies for a balanced and healthy immune maturation can be assured. Pregnancy and early childhood are particularly susceptible for exogenous influences due to the developing nature of a child’s immune system. While endogenous influences such as family history and the genetic background are immutable, epigenetic regulations can be modulated by both heredity and environmental exposures. Prenatal influences such as a mother’s nutrition, smoking, or infections influence the complex interplay of innate and adaptive immune regulation as well as peri- and postnatal influences including mode of delivery. Early in life, induction and continuous training of healthy maturation include balanced innate immunity (e.g., via innate lymphoid cells) and an equilibrium of T-cell subpopulations (e.g., via regulatory T cells) to counter-regulate potential pro-inflammatory or exuberant immune reactions. Later in childhood, rather compensatory immune mechanisms are required to modulate deviant regulation of a child’s already primed immune trajectory. The specific effects of exogenous and endogenous influences on a child’s maturing immune system are summarized in this review, and its importance and potential intervention for early prevention and treatment strategies are delineated.
Similar content being viewed by others
Introduction
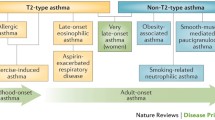
Childhood asthma can be classified into two major phenotypes, non-allergic asthma and allergic asthma [1]. While early-onset allergic asthma is most prevalent during childhood and in young adults, the non-allergic phenotype predominates in older age groups [2]. Allergic asthma is characterized by sensitization to specific allergens, high immunoglobulin (Ig) E levels, and eosinophilia, whereas non-allergic asthmatics show no sensitization and rather neutrophilic inflammation. Common triggers for asthma are among others allergen exposure, viral respiratory infections, exercise, irritants in the air, stress, and weather conditions.
Asthma is further divided into several endotypes based on pathophysiologic mechanisms. Childhood allergic asthma has been characterized by a T-helper (Th) cell type 2 – shifted endotype and decreased innate immunity gene expression. Regulatory T cells (Tregs) play a critical role in the development of childhood allergic asthma. Depending on study design, definition of Tregs, and importantly age of the children, their role is different during distinct stages of immune and asthma development. For example, Lee et al. (2007) [3] have shown a reduced number of Tregs in children with allergic airway disease, while some studies have shown increased or decreased number of Tregs at different ages in asthmatic children [4, 5]. Besides Tregs quantity, impaired function of Tregs is important for asthma development [6]. Quite distinctly, non-allergic asthma showed increased pro-inflammatory interleukin (IL)-1β/IL-17 –shifted neutrophilic inflammation and insufficient suppression of IL-5, IL-13, and interferon (IFN)-γ by Tregs [4]. In adulthood, endotyping further includes a Th2-low and Th2-high endotype, which is subdivided into early-onset allergic asthma, late-onset eosinophilic asthma, and aspirin-exacerbated respiratory disease [7]. Th2-low asthma shows no eosinophilia and a lack of response to corticosteroid therapy. It has also been linked to Th1-high and Th17-high inflammation [7].
Due to the multifactorial nature of the disease, investigations on the pathogenesis of childhood asthma are challenging. Genetic background, sex, or family history can put children at risk for asthma development inducing higher susceptibility for external triggers resulting in an imbalance of early life immune regulation.
Early childhood is a particularly vulnerable period as the immune system is still developing. A balanced interplay of innate and adaptive immune regulation is of great importance to achieve a healthy immune system in childhood and beyond. An in-depth understanding of pre- and postnatal development is crucial to further recognize modifications caused by a number of influences contributing to disease development. Once changes during early life immune regulation and their modulators are identified, possible prevention strategies need to be tailored specifically for children at high risk.
Early immune regulation: relevance of pre- and postnatal development for healthy immune regulation
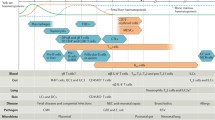
The development of healthy immune regulation already starts in utero, maturing during pregnancy, early childhood, and even in adolescence. The fetal phase and the newborn’s immunity are strongly influenced by maternal molecular and cellular components transferred to the fetus. Besides maternal antibodies also inflammatory mediators, micronutrients, microbial products, and cells are transferred. They form a complex network of multiple signals that provide immunity, program the neonatal immune system, and modulate its homeostatic regulation. There is even increasing evidence that allergens and microbial antigens can cross the placenta [8]. After birth, the newborn’s immunity has to adapt to the altered circumstances rapidly. In the first months of life, immune responses are mainly driven by components of the innate immune system including extracellular components such as the skin, spleen, mucous membranes, tissue fluids, blood, as well as secretions and cellular components including neutrophils, monocytes, macrophages, and dendritic cells (DCs) [9]. Although cellular innate immunity is present in newborns, its function of antigen presentation, phagocytosis, and cytotoxicity is not yet fully developed putting infants at risk for infections.
As first phagocytic cells in the fetus, monocyte numbers increase constantly with gestational age, although cytokine production is still reduced [10]. After birth monocytes are still low in numbers, and compared to adult cells, they are unable to fully activate antigen-specific T- and B-cell responses due to a lower expression of major histocompatibility complex (MHC) II, cluster of differentiation (CD) 80 and CD86 [11]. Some cytokines, e.g., tumor necrosis factor (TNF) and IL-6, reach comparable levels as adult monocytes by the age of 3 years, while others like IFN-γ and IL-12 do not reach adult levels until adolescence [12].
Neutrophils are present at the end of the first trimester and increase exponentially shortly before birth, exceeding even adult numbers [13]. Around 3 days postpartum, they approach adult numbers. However, the cells are still functionally impaired through a reduced ability to adhere and weaken bactericidal function caused by less production of lactoferrin and diminished chemotaxis [14].
Around gestation week 12, dendritic cell (DC)-like cells can be found in the human fetal thymus, liver, and in mesenteric lymph node. In week 23, they also populate in the fetal skin and tonsils. In cord blood, the plasmacytoid DC (pDC)/myeloid DC (mDC) ratio is 3:1 compared to a 1:3 pDC-mDC ratio in adults [11]. Low numbers of both myeloid (mDC) and plasmacytoid (pDC) DCs are present in neonates. mDCs in infants produce lower cell surface levels of human leukocyte antigen (HLA) class II, CD80 and CD86, which results in a reduced ability in priming Th1 and CD8 T-cell responses [15]. pDCs are similarly constricted as they produce low levels of IFN-α/-β upon virus exposure, therefore lacking in their anti-viral function [16].
Innate lymphoid cells (ILCs) are a relatively newly described group of cells with varying physiological functions. Group 1 ILCs include natural killer (NK) cells together with ILC1s [17]. ILC2s and multipotent progenitor type 2 cells play a role in inflammatory diseases such as asthma [13, 17]. Lymphoid-tissue-inducer cells (LTi) cells are counted among group 3 ILCs. ILCs seem to have an innate developmental program with striking parallel to that of T lymphocytes [17]. Regarding NK cells, it is suggested that their cytolytic function can be estimated by CD56. Around 50% of neonatal NK cells are CD56 negative. These cells have a reduced ability to mediate cytolysis and antibody-dependent cellular cytotoxicity in cord blood [9]. However, it is postulated that there is no intrinsic abnormality in NK cells of neonates, because stimulation with different cytokines such as IL-2, IL-12, IL-15, and IFN-γ increase cytotoxic activity to adult levels [18]. Fully functioning NK cells can be observed by 9 to 12 months of age [9].
The two components of immunity, namely, innate and adaptive immunity, are in close contact with each other. The adaptive immune responses induce specific elimination of pathogens and immunological memory. T cells are developed in the thymus, and the first mature CD4+ and CD8+ T cells appear by gestation week 15 [19]. Regarding T-helper cell immunity, there is a strong shift toward type 2 immunity [20]. Although in the first trimester of pregnancy, high levels of pro-inflammatory IFN-γ ensure maintenance of pregnancy, in the following phase, a rather weak function of Th1 cells results in low IL-12 secretion, which stimulates NK cells and induces Th1-type effector cell differentiation from naïve CD4+ T cells [21]. Hence, less IFN-γ is produced, rendering neonates vulnerable to certain infections [21]. This shift found in newborns can potentially be explained by the fact that pro-inflammatory cytokines induce spontaneous abortion, and the immune reaction is characterized by tolerogenic reactivity [22]. At birth, almost all T cells carry the CD45RA glycoprotein being typical for naïve T cells, which have never encountered foreign antigens. The number of Tregs within the CD45RA-negative T cells is relatively high but decline during childhood, and Th1, Th17, and Th2 cells increase to approximate the same number of naïve T cells [23]. Fetal naïve CD4+ cells tend to develop toward forkhead box P3 (Foxp3) + CD25+ Tregs in response to alloantigens promoting self-tolerance. Peripheral Tregs add up to about 3 % of the total CD4+ T cells at birth. They exist for an extended period of time to provide an anti-inflammatory profile to early life immune responses [23]. In parallel to high production of T cells, the thymus reaches its maximum size in the first years of life [19].
B lymphocytes are detectable in the fetal liver and omentum as early as 8 weeks of gestation, and they first appear in fetal blood circulation by 12 weeks of gestation and in the fetal spleen by 13 weeks of gestation. B-cell receptors continue to diversify with advancing gestational age [24].
Although newborns’ B-cell numbers are comparable to adult levels, approximately 95% of the total B cells are naïve compared to about 10% in adults [25]. There is a lack of plasma cells and a low IgM concentration, which increases rapidly in the first month. Since maternal IgG is transferred across the placenta, neonatal IgG levels are remarkably high at birth but decrease within the first 3 months due to metabolization of maternal IgG before IgG production starts, resulting in a physiological hypogammaglobulinemia of infancy [26]. At birth, there are also low serum levels of IgE and IgA [8]. IgE might be transferred to the fetus as IgG/IgE complexes [8]. The breastfed infant also receives IgA and IgM transferred by polymeric immunoglobulin receptors across mammary epithelial cells [8]. Those antibodies are not absorbed by the blood circulation of the neonate but serve an immunosuppressive role, prevent inflammation by oral antigens, and shape the composition of the gut flora. The IgG levels reach about 70% of adult levels by 1 year of age. IgA reaches at this time point only 30% of adult levels [27].
Impaired immune regulation during childhood asthma development
There are several different hypotheses regarding altered immune development leading to childhood asthma including the so-called hygiene hypothesis, the two-hit hypothesis, and a hypothesis regarding viral infections. It is well-known that the risk for asthma is magnified if sensitization is manifested in very early childhood. Therefore, allergy development is a major focus of all hypotheses.
Generally, it is suggested that a prolonged shift toward Th2 immunity is associated with an increased risk for sensitization and for severe respiratory infections. This can result in airway inflammation at a sensible stage during lung development and growth. A prolonged Th2 shift could be the consequence of an already existing predisposition; however this has not been clarified yet. A number of immune changes early in life indicate “priming” for allergic diseases. Those comprise higher IgE levels in cord blood and less capacity of peripheral blood mononuclear cells (PBMCs) to produce IFN-γ at birth in infants with an atopic family history [28]. Furthermore, external prenatal influences by maternal allergen exposure result in a stronger Th2-biased response [29]. This response may be reinforced by continuous exposure to the same antigens in the first months of life, leading to persistent IgE responses.
The hygiene hypothesis postulates that improved hygiene decreases exposure to bacterial and parasitic infections resulting in reduced Th1-shifted immune development [30]. A proper balance between Th1 and Th2 is important in healthy immune regulation. Newborns generally express a Th2-skewed immunity, and in the absence of immune-maturing infections, young children remain inclined to the development of Th2 responses and may develop atopic disease [31]. According to this hypothesis, Th1-driving infections and the induction of innate immunity can educate immune response in neonates and infants [30]. However enhanced Th2 immunity associated with allergic disease has been questioned, e.g., influenza infections can under specific conditions enhance allergic disease caused by Th1-polarized DCs that produce IFN-γ [32].
The two-hit hypothesis postulates that asthma inception requires both a genetic predisposition (first hit) together with a second hit, e.g., a severe viral infection. If only one factor is present, the course of the allergic response or the infectious disease may only be transient [33]. Respiratory syncytial virus (RSV) or Rhinovirus (RV) infection in early childhood has been associated with an increased risk of asthma that may persist into adulthood. It is not clear yet whether bronchiolitis caused by the virus infection leaves lung injury that results in wheeze episodes and asthma development or if an inherent predisposition facilitates acute bronchiolitis and subsequent asthma [34].
A more recent hypothesis may include the concept of trained immunity. In the past, only the adaptive immune system was ascribed, the ability to adapt to certain triggers. Recently, it has been shown that the innate immune system can respond to reoccurring stimuli via memory function [35]. In order to protect the body from excess reactions to harmless stimuli and to avoid chronic inflammation, negative regulators constantly compensate inflammatory processes. These anti-inflammatory regulators might be increasingly active upon identification of known harmless antigens as innocuous by means of innate immune function. In general, gene expression of asthmatic children is detrimentally shifted toward pro-inflammation with harmful consequences like tissue destruction. Recently, it was shown that asthmatic children express lower levels of anti-inflammatory TNFAIP3 (A20) that negatively regulates several inflammatory mediators of the NF-κB pathway [36].
Influences on early immune regulation: Role of endogenous influences
Importance of the family history and the sibling effect
The pathophysiology of asthma is influenced by a complex interaction of genetic and environmental factors (Fig. 1). Family history is one of the most important parameters for allergy prediction in the clinic and is the strongest risk factor for lifetime asthma prevalence [37]. Allergic asthma is characterized by a polygenic hereditary predisposition to excessive IgE production. In fact, if one parent is atopic, the recurrent child’s risk for asthma is about 25%. While maternal asthma results in early asthma development, paternal asthma affects asthma development later in life with declined asthma risk over time. In contrast, when both parents are asthmatics, the child’s risk for asthma development is about 50%, increasing over time [38]. Moreover, the number of asthmatic siblings seems to influence the child’s asthma risk in an independent and dose-response pattern [38]. Genetic variations might influence several endotypes of asthma to different degrees, with a high association of family history with early-onset asthma in contrast to late-onset asthma [39]. In addition, also the severity of the disease seems to have a genetic component, e.g., the hedgehog-interacting protein gene (HHIP)/rs1512288 variant was identified as a significant predictor of forced expiratory volume (FEV) 1 and forced vital capacity (FVC) in asthma, and an increasing number of risk variants in these lung function genes were highly associated with increased asthma severity [40].
Besides these hereditary factors, also non-genetic influences of family composition have an influence on the child’s asthma risk. David Strachan published already in 1989 that there is a highly significant inverse link between hay fever and the number of older siblings [30], replicated in several studies. The assumed immunological mechanisms underlying the epidemiological findings involve in utero programming and altered immune maturation as less hygienic conditions caused by a larger household contribute to asthma protection which are summarized in the concept of the hygiene hypothesis. In fact, birth order seems to affect both humoral and cellular responses prenatally. A study investigating IgE levels in cord blood samples could demonstrate reduced levels of IgE with increasing sibling numbers with an odds ratio (OR) of 0.78 for the second and 0.57 for the third child, respectively [41]. Also the responsiveness of cord blood mononuclear cells (CBMCs) to harmless allergens is negatively associated with the number of older siblings [42]. In early childhood, enhanced exposure to microorganisms and more frequent infections associated with a higher number of siblings confer the protective effects inter alia by differential colonization pattern of both the skin and gut. In contrast to children with older siblings, firstborns are primarily colonized by Clostridium difficile associated with higher asthma risk, while the colonization rate of species associated with asthma protection like Lactobacilli and Bifidobacteria was rather low [43]. Moreover, the increased exposure to a wide range of microorganisms correlated with more siblings supports the switch to a Th1-mediated immune response counter-regulating the development of allergic Th2 immunity. In contrast, in severe forms of asthma and eczema, there was a positive association with the number of siblings [44].
Role of genetics
Since the first genome-wide association study (GWAS) of asthma in 2007, variants in several loci have been linked with asthma. Especially, single-nucleotide polymorphisms (SNPs) in the 17q21 locus and its flanking regions (17q12-21) are replicated robustly to be associated with asthma risk in childhood-harboring IKAROS family zinc finger 3 (IKZF3), zona pellucida-binding protein 2 (ZPBP2), gasdermin B (GSDMB), and orosomucoid-like 3 (ORMDL3) [45]. These genes are involved in multiple important processes like gene transcription, apoptosis, and sphingolipid synthesis. Importantly, also the calcium homeostasis in the endoplasmic reticulum is regulated by ORMDL3 with important consequences for T-cell activation and inflammation [46]. Since newborns carrying the genetic risk variant within the 17q21 locus showed significantly increased secretion of IL-17, a functional role of this locus on Th17 cell development during early immune maturation is suggested [47]. In a recent study comparing risk allele carriers in 17q21 SNPs, increased wheeze risk was shown to be mediated by immunological mechanisms including downregulation of toll-like receptor (TLR) TLR2 gene and IL-2 cytokine and upregulation of ORMDL3 gene and IL-17 cytokine in the subsequent wheeze phenotypes [48]. Moreover, increased expression of ORMDL3 has been shown to induce CXC and CC chemokines involved in the recruitment of T cells and mucus production and Th2 cytokines like IL-4 and IL-13.
Besides this prominent locus, SNPs in 1q31-32 (IL-10), 2q12 (IL-1RL1/IL-18R1), 5q22 (thymic stromal lymphopoietin (TSLP)), 5q31.1 (interferon regulatory factor 1 (IRF1)), 6q21 (human leukocyte antigens (HLA)-DR), and 9p24 (IL-33) have been identified as asthma susceptibility loci [49]. Their immunological role involves primarily the regulation of Th1/Th2 immunity (IL10, IL-1RL1, IL-18R1, TSLP, IL33, IRF1) and antigen presentation (HLA-DR). By investigating genetic variations in subphenotypes of asthma, risk alleles in the receptor for Rhinovirus C (cadherin-related family member 3 (CDHR3)) at the 7q22.3 locus were identified to be associated with increased early-onset asthma with acute exacerbations [50].
Environmental influences like smoking and viral infections have been shown to modulate the effect of the genetic variants within the 17q21 locus on asthma development [51, 52]. Early life exposure to cat allergens influences the asthma risk for carriers of certain SNPs in this locus [53]. Highly complex gene-environment interactions have been shown to influence the susceptibility for asthma development, potentially explaining why some children do not develop symptoms although carrying risk variants for asthma-associated genes. Thus, environment-driven protective measures could be applied in the clinic to prevent asthma development in high-risk children.
Epigenetic influences
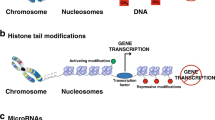
Besides genetic variations, also modifications in gene expression by epigenetic regulation without changes in the deoxyribonucleic acid (DNA)-sequence influence the pathophysiology of childhood asthma. Especially DNA methylation and histone modifications are important epigenetic processes involved in asthma development [54].
Genomic imprinting, monoallelic expression of genes due to epigenetic silencing dependent on parental origin, and oocyte-specific histone tail modifications are directly heritable epigenetic regulations. In this manner, polymorphisms in FCεR1-β linked with asthma and atopy development are only inherited by the mother [55]. In addition, indirect genetic components of epigenetic regulation have been shown to contribute to asthma development.
Particular DNA methylation patterns could be observed in CBMCs of newborns that developed asthma later in life. Specifically, an inverse association with DNA methylation of Th2 genes, including GATA-binding protein 3 (GATA3), IL-4R, and T-box transcription factor 21 (TBX21), as well as the IL-33 receptor ST2, and the risk for asthma and other atopic diseases, was described [54]. The resulting induction of CD4+ T-cell differentiation into Th2 T cells leading to enhanced secretion of Th2 cytokines like IL-4 and IL-13 further contributes to the asthmatic phenotype. In fact, prominent Th2 cytokines were less methylated in asthmatic patients, resulting in higher expression of these asthma-promoting mediators. Interestingly, also gender-specific methylation structures were found, as CpG hypermethylation of the receptor tyrosine kinase AXL in newborns blood was associated with wheeze at age 6 especially in girls [56]. Epigenome-wide association studies (EWAS) revealed consistently hypomethylated signatures driven by higher numbers of eosinophil, a granular cell type that is known to be centrally involved in allergic inflammatory processes [57]. Moreover, low methylation and subsequent overexpression of T-cell developmental genes including runt-related transcription factor 3 (RUNX3) and T-cell immunoreceptor with Ig and Immunoreceptor tyrosine-based inhibitory motif (ITIM) domains (TIGIT) have been demonstrated in asthmatics [58]. While the transcription factor RUNX3 mediates blood cell maturation of hematopoietic stem cells, the immune receptor TIGIT expressed on T cells, NK cells, and DCs regulates cytokine secretion resulting in inhibition of T-cell activation.
Although there is a clear hereditary component to epigenetics, environmental exposures also lead to changes in epigenetic markers both pre- and postnatal [59]. For example, exposure to particulate matter has been shown to be associated with DNA methylation of genes involved in antigen presentation (HLA-DOB) and cytokines, e.g., IL-10 and IL-13 [60]. In contrast to IL-13 promoting asthma development by stimulating B-cell differentiation, IL-10 contributes to the prevention of inflammation by suppressing pro-inflammatory cytokines like IL-1, IL-5, and granulocyte-macrophage colony-stimulating factor (GM-CSF) and inhibition of T-cell co-stimulation. Also prenatal exposure to cigarette smoke has an impact on DNA methylation including innate immune genes like CD14, the co-receptor of the pattern recognition receptor complex TLR4 and MD-2, which is required for lipopolysaccharide (LPS)-mediated signaling [61].
Since epigenetic modifications are dependent on both genetics and the environment, epigenetic regulation might display the link between these two important players.
Exogenous influences on early immune regulation
Although endogenous influences are indeed of high importance for allergic diseases, the rapid increase of childhood asthma prevalence during the last five decades and the prominent rural-urban differences emphasize the impact of external factors. Environmental exposures can be classified in predominantly protective and harmful factors, while some environmental triggers can be classified in both groups dependent on their intensity, duration, frequency, and time of exposure.
Prenatal influences on early immune regulation
Environmental factors
The strongest asthma protective factor described so far is the farm environment. Prenatal exposure to stables and animal contact was shown to be inversely associated with atopic diseases. Neonates with prenatal exposure to farming environments showed increased innate immunity-associated gene expression including TLR2, TLR4, and CD14 associated with asthma protection later in life. Moreover, CBMCs of these children showed higher numbers and more efficient Tregs [62]. By interaction with DCs via suppressive co-stimulatory molecules and cytokines like IL-10, IL-35, and transforming growth factor (TGF)-β, Tregs can induce inhibition of effector T cells. Moreover, by granzyme-perforin-dependent signaling, Tregs can even directly eliminate T lymphocytes. Also, IgE and cytokine production are modulated by maternal farm exposure [63, 64].
Besides the protective effects of farms, also harmful influences of cities like traffic-related air pollution modulate fetal immune regulation and increase asthma risk due to impaired lung function [65]. Moreover, higher exposure to air pollution in utero results in lower levels of T lymphocytes, while B cells and NK cells are enhanced in CBMCs [66]. Also, the production of important cytokines was modulated with impaired IL-10 secretion and increased IL-1β levels [67]. Early life exposure to cigarette smoke is associated with highly detrimental consequences for the child. The harmful effects on asthma development are not only limited to the direct exposure to the toxins in the uterus but also morphological alterations and decreased blood circulation due to less nutrient intake. Reduced growth of the child accompanied by delayed lung development, a higher premature birth rate, and more complications at birth contribute to increased asthma risk in children with prenatal exposure to cigarette smoke. Immunological mechanisms involve impaired Th1-type responses with a shift to Th2 immunity together with enhanced secretion of pro-inflammatory cytokine IL-17A by NK, NKT, and γδ T cells induced by exposure to cigarette smoke [68]. Accompanied by increased oxidative stress and imbalanced compensatory antioxidants, enhanced inflammation with increased expression of IL-8, IL-6, and TNF-α results in chronic mucosal inflammation. Moreover, mucus overproduction, excessive recruitment of macrophages and neutrophils, and increased permeability of epithelial cells are reported consequences of paternal smoking [68].
Maternal nutrition and stress
Fetal nutrient uptake plays an important role in the development of numerous diseases. In general, a balanced and Mediterranean diet is advised to prevent allergy development of the child [69, 70]. Especially a high intake of omega-3 fatty acids found in fish oil showed anti-inflammatory effects in CBMCs and reduced oxidative stress in the placenta along with reduced risk for allergic diseases later in life [71]. Although vitamin D is positively associated with protection of wheeze and eczema, it is yet still under debate. Recently, the positive effects of vitamin D supplementation during the second and third trimester of pregnancy on responsiveness on innate and mitogen stimuli by upregulation of cytokine production and TLR2 and TLR9 expression were shown in newborn CBMCs. Also IL-17A production was increased upon the stimulation of T cells which was associated with immune defense mechanisms against pathogens [72]. Moreover, maternal nutrition has important influences on the child’s microbiome via nutrients in breast milk, with further effects on the prevention and development of childhood asthma [73].
The health outcome of a child has been shown to be additionally influenced by the psychological and social stress of the mother during pregnancy. Anxiety and depressive symptoms with altered cytokine, growth factor, and hormone levels can influence fetal programming via several mechanisms. Besides the indirect effects including preterm birth and low birth weight, maternal prenatal stress (MPS) can also directly increase the risk of asthma and other immunological diseases of the child. In addition to Th2 shifting and attenuated activity of NK cells and macrophages, also modifications in the T-cell response to specific antigens contribute to the association of MPS and childhood asthma development [74]. Moreover, impaired lung development as a consequence of prenatal stress was shown sex specifically in mouse models [75].
Infections and antibiotics
Especially maternal respiratory and urogenital infections are positively associated with asthma development of the child later in life. Respiratory infections in the first trimester of pregnancy, where strong inflammatory conditions predominate the immunological status, are particularly relevant for subsequent asthma development [76]. Any interference with this highly sensitive and coordinated process could result in severe impairments of the developing immune system or even in pregnancy aborts. Especially pro-inflammatory processes and cytokine production accompanied by infections have a negative impact on the lung function of the child [77]. Despite the direct effect of prenatal infections, indirect consequences contribute also to the detrimental effects of maternal infections including preterm birth, low birth weight, and maternal antibiotic use that are highly correlated with childhood asthma development [78]. In addition, the frequent use of antibiotics during pregnancy is associated with a higher risk for wheeze and asthma development later in life. A meta-analysis revealed a positive association between antibiotic use and the risk of wheeze and asthma (OR 1.28) [79]. Since antibiotics influence the maternal bacterial composition, the microbiome transferred to the newborn during delivery might have a high impact on the development of asthma due to alterations in the Th1/Th2 immune balance [80].
Perinatal influences on early immune regulation
There is a high increase in cesarean sections, although adverse effects are known on the development of a variety of childhood diseases. Several studies have shown that cesarean section is associated with an increase in asthma risk of about 20% [81,82,83]. This effect is assumed to be driven by the differing microbial exposures during delivery, since postnatal bacterial colonization is decisive for maturation of the immune system. Children born by cesarean section are colonized by bacteria originating primarily from the hospital environment and the skin, delayed colonization of commensal gut bacteria like Bifidobacteria [84], and increased levels of asthma-associated cytokines IL-13 and IFN-γ [85]. In case of an emergency cesarean section, prematurity is also responsible for multiple detrimental effects on newborns’ health. Specifically, for asthma development, the asthma risk for children born preterm is highly increased with an OR of 4.06 (95% confidence interval (CI) 3.59–4.59) [86]. Low birth weight and reduced fetal size are also some indirect effects of prematurity on asthma development mediated by the negative association with risk for childhood asthma and general poorer lung function.
Postnatal influences on early immune regulation
Role of the Environment
Numerous epidemiological studies worldwide have repetitively confirmed a significantly reduced prevalence of childhood asthma and allergies in rural areas and especially farming environments, often referred to as “farm effect” [87]. In particular, the exposure to stables and consumption of raw milk with high bacterial diversity seem to convey protection. A recent study demonstrated the asthma-protective role of farm-associated bacterial composition, since asthma risk was reduced in non-farm children, when the bacterial microbiota composition of their home were similar to that of farm homes [88]. These protective effects were attributed to innate immune activation (e.g., TLR2), balanced immune regulation via DC and Treg-cell regulation, and IFN-γ secretion [5, 89,90,91].
Two more environmental exposure studies in the USA (Amish people) and China reported comparable or even stronger “allergy-protective” effects [92]. The low prevalence of asthma at school age of Amish (5.2%) vs Hutterite children (21.3%) – both with similar genetic background and large family size – may be related to difference in farming style, with traditional techniques in the Amish and modern, highly industrialized farming in the latter [93, 94]. These differences in farming style were also mirrored in the levels of endotoxin, a cell wall component of Gram-negative bacteria, with 6.8-fold-higher endotoxin levels in Amish house dust samples [95]. Moreover, not only farming but also even rural areas have been shown to convey asthma-protective properties. In a study investigating rural and urban areas in China, a significantly reduced childhood asthma prevalence in rural (2.8%) vs urban (29.4%) China associated with significantly higher endotoxin levels in house dust was observed [96].
In contrast to children growing up in urban areas, children from farming environments spend significantly more time outdoors with consequently more exposure to allergens. Moreover, regular contact to farming animals and consumption of raw milk in this microorganism-rich environment results in constant stimulation of the immune system. To avoid excessive reaction against these mostly harmless stimuli, adaptive immune tolerance mechanisms are initiated by repetitive stimulation.
Especially tolerance against endotoxin has been extensively researched. Upon repetitive and prolonged stimulation with this bacterial cell wall component, the immune system responds in a significantly attenuated manner and even directly downregulates pro-inflammatory signaling. Recently, anti-inflammatory capacities of farm dust stimulation could be shown ex vivo in children by the upregulation of anti-inflammatory tumor necrosis factor alpha-induced protein 3 (TNFAIP3). Its gene expression was even decreased in CBMCs of healthy newborns, developing asthma later in life [36]. In addition, pro-inflammatory genes like TLR4 and myeloid differentiation primary response 88 (MyD88) have been significantly downregulated upon farm dust stimulation in urban asthmatic children from Europe and China. With their role in compensating inflammatory processes, Tregs are highly involved in the protective effects. Newborns with farming exposure in utero had increased levels of Tregs in cord blood [62]. For 4.5-year-old children, Treg numbers were significantly increased in farm children and were negatively associated with asthma development [90]. However, an immunological switch results in lower Treg levels in farm children at school age [5]. Farm exposure has also been associated with less numbers of DCs [91, 97] that are in first immunological contact with the allergens and decide about following T-cell response.
There are also harmful environmental exposures like traffic-related air pollution or fungal exposure contributing to the development of childhood asthma. Ambient pollutants caused by motor vehicles are primarily black carbon, nitrogen dioxide (NO2), carbon monoxide (CO), and particular matter (PM) with an aerodynamic diameter of less than 2.5 (PM2.5) or 10 μm (PM10) which were associated with reduced lung growth and lower lung function in a dose-dependent matter [98]. Their participation in childhood asthma development is estimated at 14% [99]. Also indoor pollutions, e.g., deriving from combustion of biomass, were correlated with the development of wheeze and asthma in children [65]. The underlying immune mechanisms involve increased oxidative stress, resulting in damaged integrity of epithelial barriers and pro-inflammatory processes [100]. Moreover, a Th17 and Th2 shift upon exposure to PM and diesel exhaust particles (DEPs) has been shown with increased Th2 cytokine secretion and production of specific IgE in mouse models [101].
Mold contamination is another risk factor for allergic and non-allergic asthma, although the potential of fungal allergens for sensitization is rather low. Especially the pathogen-associated molecular patterns (PAMPs) including chitin and β-glucan trigger inflammatory responses with detrimental effects. In mouse models, the induction of IL-25, IL-33, TSLP, ILC2 cells, and Th2 immune response was shown for the inner cell wall component chitin [102]. Moreover, the outer cell wall component β-glucan has been demonstrated to enhance airway hyperresponsiveness (AHR) and promote airway inflammation [103]. Besides these PAMP-triggered innate immune processes, fungal proteolytic allergens contribute to the harmful effects.
Influence of Pets
There are conflicting data concerning the influence of exposure to cats on the development of asthma in childhood. Investigations are especially complex since families with increased risk avoid possibly allergenic sources. Sensitization against cat allergens might be dependent on the time point, type of allergenic protein, and the interaction with other risk factors. Especially cat exposure in preschool age is associated with higher risk for sensitization against cat allergens, while by the age of 16, no difference between adolescents with and without cat exposure could be observed [104]. In two Swedish cohorts, a reduction in risk for allergy development was recently shown [105]. This protective effect might be mediated by a higher diversity in bacterial composition of house dust in line with the concept of protective farming environments [106].
Breastfeeding and nutrition
Due to the multiple health benefits, breastfeeding is recommended from the World Health Organization (WHO) for at least the first 6 months up to 2 years [107]. The German Academy of Pediatrics and Youth Medicine (DGKJ) recommends breastfeeding for at least 4 months with subsequent gradual introduction of other foods. In case of inadequate breast milk, the European Academy of Allergy and Clinical Immunology (EAACI) recommends the use of hypoallergenic formula milk for newborns with a high risk of allergies [108]. Some studies suggest protective effects of breastfeeding on the development of childhood asthma, but there are conflicting data published [109]. Beneficial effects for the infant’s immune system include immunoglobulins like IgA, anti-microbial enzymes, cytokines and chemokines, and polyunsaturated fatty acids (PUFAs) [110]. In addition, the bacterial diversity is increased in breastfed children, which is by itself positively associated with asthma and allergy protection [111]. A positive correlation of breastfeeding and protection against early respiratory infections was shown that might indirectly mediate asthma protection [112].
A balanced diet is generally recommended. To maintain the balance between oxidants and antioxidants, the intake of zinc as a co-factor for enzymes to cope with oxidative stress is important. By effects on certain immune cells including epithelial cells, T cells, B cells, macrophages, and DCs and stimulatory effects on macrophage recruitment and anti-microbial protein production, vitamin D beneficially modulates immunological processes [113]. In addition, the consumption of PUFAs that are converted to eicosapentaenoic acid and docosahexaenoic acid contributes to asthma protection by conversion of anti-inflammatory lipid mediators like resolvins and protectins. Contrary to earlier recommendations, the avoidance of potentially allergenic foods is no longer advised, since early introduction of potentially allergenic food like egg or peanut enhances immune tolerogenic effects. However, diverse data in the association of nutrition and breastfeeding on asthma development require more studies to elucidate immunological mechanisms.
Infections and the microbiome
Distinct types of infections were suggested as asthma-protective, while others were highly positive correlated with childhood wheeze and asthma. Acute respiratory tract infections (ARIs) are triggered predominantly not only by RSV and RV, but also influenza virus, coronavirus and parainfluenza virus are significant risk factors for asthma development in children, depending on the load and timing of exposure. Although up to 50% of all children suffer from ARI-associated wheeze, it is yet unknown why some of them develop asthma, while others restore their healthy status [114]. While viral infections shift the immune response to the Th2 side, anti-viral response carried out by mediators of the Th1 axis is reduced [34, 115]. Moreover, dysregulation of epithelial gene expression increased inflammation, and decreased inhibition of viral replication was shown [116].
“Asthma-protective” species include Toxoplasmosis gondii and Helicobacter pylori. While T. gondii infection triggers IFN-γ production, reduced Th2 immunity, and higher proliferation of allergen-specific T cells [117], the neutrophil-activating factor of H. pylori suppresses Th2 response by upregulating IFN-γ, TNF-α, IL-12, and IL-23 secretion by neutrophils, monocytes, and DCs [118]. Moreover, helminth infections seem to reduce asthma and allergy risk by suppressing the hosts immune system involving Tregs, B cells, and modulations of macrophages and DCs. The resulting hyporesponsiveness leads to anti-inflammatory processes by enhanced IL-10 and TGF-β production [119].
The composition of the gut microbiome is important for healthy immune development, and initial colonization seems decisive [120]. While maternal exposures influence the child’s microbiome during pregnancy, birth and external exposures in the first 3 years of life are critical for the maturation of the gut microbiome postnatally. Dysbiosis and an impaired gut microbiome contribute to the development of multiple diseases [121]. A recent study investigating the gut microbiome of 690 1-year-old children revealed that children with an immature microbial composition have an increased asthma risk at the age of 5 years [122]. Lactobacillus and Bifidobacteria were shown to be protective for asthma and allergy development and are associated with vaginal birth, breastfeeding, and early contact to farming animals. In fact, in human peripheral blood of volunteers administered with Bifidobacterium infantis, increased expression of the Treg markers IL-10 and Foxp3, dependent on TLR and indoleamine 2,3-dioxygenase (IDO) expression by DCs, was found. Moreover, Bifidobacterium breve has been associated with reduced airway hyperresponsiveness, Th2 activation, eosinophilia, and allergen-specific antibodies including IgE and IgG1 [123].
Also for Clostridia species, whose levels are increased by vaginal delivery, enhanced development of Tregs has been shown by the generation of short-chain fatty acids [123]. In contrast, for Staphylococcus, Streptococcus, and Bacteroides, primarily harmful properties with increased risk for allergy development have been demonstrated. While Streptococcus is frequently found in urban environments, they are less abundant in home dust samples of farming families [88]. These asthma-protective effects of bacterial components within the farming environments are at least partly driven by reduced secretion of pro-inflammatory cytokines like IFN-γ, IL-1β, IL-6, and IL-12 [88]. Also, bacterial richness and diversity are positively correlated with asthma protection [124]. Comparing nasal microbiota of healthy and asthmatic children, a significantly decreased α and β diversity was shown for children suffering from asthma [125].
Modulation of immune responses: early modulation for prevention
Primary prevention as central factor
As the onset of allergy occurs usually during early childhood, primary prevention needs to be implemented early in life. Whether only high-risk children with a family history of atopic disease should be included into the measures is still a matter of debate and depends on the safety and cost of possible options.
One way to implement primary prevention of asthma may be the reduction of immunologic sensitization of young children [126]. While some primary prevention options have been tried more or less successfully for years, e.g., avoidance of tobacco smoke, allergen exposure, and nutrition, a window of “healthy immune regulation” not only in terms of timing but also in terms of “variability and flexibility” of immunity has still not been defined. Whether only avoidance of immunological sensitization is key to primary prevention is open. Potentially, keeping early life immunity trained efficiently – equipped with immediate options to keep it in balance – may be dependent on specific influences.
It was speculated that application of anti-IgE antibodies reduces the high-affinity receptor for IgE expression on mast cells, basophils, and DCs, e.g., via reduction of free IgE levels [127], and may be able to prevent allergic sensitization and development of allergic asthma. Yet a practical, affordable approach is not in sight. How interventions aiming to improve children’s response to viral infections by inhaled IFN-β and TLR agonists are convertible is similarly open.
Examples of secondary prevention and tertiary prevention
Secondary prevention strategies try to reduce the incidence of clinical manifestations such as allergic rhinitis or asthma in already sensitized children with first clinical symptoms.
One approach shown to be effective in improving symptoms of respiratory allergies is allergen immunotherapy which is either applied subcutaneous (SCIT) or sublingual (SLIT), with multiple beneficial effects on immune regulation. On a cellular level, a reduction in the activity of mast cells and basophils upon therapeutic allergen exposure is reported. Specifically, basophil inhibition is mediated by suppression of the histamine receptor 2 [128]. Moreover, Tregs and regulatory B cells (Bregs) are increased, resulting in enhanced IL-10 secretion, inducing T-cell tolerance together with TGF-β, cytotoxic T-lymphocyte-associated protein 4 (CTLA4), and programmed cell death 1 (PD1). In addition, the production of IgG4 is increasingly activated resulting in enhanced inhibition of its competing antibody IgE [128]. The late-phase response of allergic reactions is also diminished upon immunotherapy with subsequently reduced recruitment and activation of eosinophils and neutrophils.
Although some studies have demonstrated safety and efficiency of specific immunotherapy for children from 3 years, the European Medicines Agency’s Paediatric Committee does not recommend this therapy before the age of 5 years. Studies for secondary prevention early in life are currently evaluated.
Tertiary prevention aims to reduce permanent damage to the lung and other organs in patients with existing asthma and/or allergy diagnosis. Tertiary prevention is mostly mediated through drugs and thus further investigated in the following section.
Late modulation: option of reversibility
The conventional asthma therapy uses a stepwise decision model, with the aim to find the minimal treatment level that controls symptoms effectively and reduces future risk. These therapeutic approaches are mainly based on the use of short-acting beta 2 agonists (SABAs), inhaled corticosteroids (ICSs), and long-acting beta 2 agonists (LABAs) in combination with ICS, with effects in muscle relaxation and bronchodilation, suppressed inflammation, and airway hyperresponsiveness in a non-specific fashion [129]. Other options for patients with persisting symptoms include leukotriene receptor antagonists that block the activation of inflammatory processes, anti-muscarinics, and/or oral corticosteroids in severe cases [129].
These treatment regimens are rather uniform for a large number of patients. Yet, more patient-tailored treatments may improve symptom control and treat the small number of severe asthmatic children more efficiently. Thus, several new approaches have been introduced as immune-regulatory therapy of severe asthma, first in adults. A number of monoclonal antibodies (mAbs) have been created to block biologic targets such as IgE, IL-5, IL-13, IL-4, and TSLP [129]. Omalizumab, an anti-IgE mAb, approved for allergic asthma in children from age 6 years on, blocks free IgE, thereby restricting the interaction with immune cells that promote allergic inflammation. Anti-IL-5 therapies target maturation, activation, and survival of eosinophils. Mepolizumab and reslizumab mAbs for IL-5 and benralizumab, an anti-IL-5 therapeutic, target the IL-5 receptor and can thus diminish eosinophilic inflammation [129]. Many other phase II and phase III studies regarding biologics are still ongoing with promising outcomes [130]. Whether and which of these, besides anti-IgE and anti-IL-5, will realistically be applicable for childhood asthma is still open.
Conclusion
Early immune regulation is one critical facet to keep healthy immune development in balance to avoid exuberant regulation toward disease-promoting immune pathways. Numerous influences at different times of immune maturation have their impact during childhood asthma development and highlight the multifactorial nature of its pathogenesis. The immune system itself is influenced by an abundance of environmental and nutritional factors starting already in utero. These include exposures to different allergens and bacterial components with their effects on various immune cells including eosinophils, neutrophils, epithelial cells, DCs, and Tregs. Endogenous factors influencing early immune regulation involve genetic and epigenetic factors that determine susceptibility for asthma. Prenatally, especially maternal environmental exposures including maternal nutrition, infections and the use of antibiotics affect the development of allergy by interfering with the maturation of the child’s immune system. Pre- and postnatally, environmental exposures like farming environments and also air pollutants and contact to pets shape the still developing immune system of the child by regulating cell differentiation, cytokine secretion, and balancing T-cell immunity. Moreover, dietary components and early life infections are associated with microbial colonization pattern in the child that are either associated with protection or increased risk for asthma development.
While this multidimensional nature of disease development makes its understanding complex, it may still offer preventive and therapeutic options at several stages of immune development and during early symptom development. While it is critical that central pathways in asthma pathogenesis are targeted, the hope is that early prevention will become possible to keep healthy immune maturation in balance. Actually, the flexible and reversible quality of early life immunity offers a promising chance to intervene, if children at risk are closely and thoroughly supervised by specialized physicians trained for prevention. The best and most specific option for distinct patient groups still needs to be established.
References
Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW (1999) Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 160(3):1001–1008
Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE (2004) Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol 113(1):101–108
Lee J-H, Yu H-H, Wang L-C, Yang Y-H, Lin Y-T, Chiang B-L (2007) The levels of CD4 + CD25+ regulatory T cells in paediatric patients with allergic rhinitis and bronchial asthma. Clin Exp Immunol 148:53–63
Raedler D, Ballenberger N, Klucker E, Böck A, Otto R (2015) Prazeres da Costa O et al. Identification of novel immune phenotypes for allergic and nonallergic childhood asthma. J Allergy Clin Immunol 135(1):81–91
Schröder PC, Illi S, Casaca VI, Lluis A, Böck A, Roduit C, Depner M, Frei R, Genuneit J, Pfefferle PI, Roponen M, Weber J, Braun-Fahrländer C, Riedler J, Dalphin J-C, Pekkanen J, Lauener R, von Mutius E, Schaub B, the PASTURE study group (2017) A switch in regulatory T cells through farm exposure during immune maturation in childhood. Allergy 72:604–615
Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, Griese M, Krauss-Etschmann S (2007) Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol 119:1258–1266
Kuruvilla ME, Lee FE-H, Lee GB (2019) Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin Rev Allergy Immunol 56(2):219–233
Jennewein MF, Abu-Raya B, Jiang Y, Alter G, Marchant A (2017) Transfer of maternal immunity and programming of the newborn immune system. Semin Immunopathol 39(6):605–613
Dalal I, Roifman C 2019 Immunity of the newborn. UpToDate.
Hallwirth U, Pomberger G, Pollak A, Roth E, Spittler A (2004) Monocyte switch in neonates: high phagocytic capacity and low HLA-DR expression in VLBWI are inverted during gestational aging. Pediatr Allergy Immunol 15(6):513–516
Basha S, Surendran N, Pichichero M (2014) Immune responses in neonates. Expert Rev Clin Immunol 10(9):1171–1184
Yerkovich ST, Wikström ME, Suriyaarachchi D, Prescott SL, Upham JW, Holt PG (2007) Postnatal development of monocyte cytokine responses to bacterial lipopolysaccharide. Pediatr Res 62(5):547–552
Nussbaum C, Gloning A, Pruenster M, Frommhold D, Bierschenk S, Genzel-Boroviczény O, von Andrian U, Quackenbush E, Sperandio M (2013) Neutrophil and endothelial adhesive function during human fetal ontogeny. J Leukoc Biol 93(2):175–184
Filias A, Theodorou GL, Mouzopoulou S, Varvarigou AA, Mantagos S, Karakantza M (2011) Phagocytic ability of neutrophils and monocytes in neonates. BMC Pediatr 11:29
Willems F, Vollstedt S, Suter M (2009) Phenotype and function of neonatal DC. Eur J Immunol 39(1):26–35
Schüller SS, Sadeghi K, Wisgrill L, Dangl A, Diesner SC, Prusa AR, Klebermasz-Schrehof K, Greber-Platzer S, Neumüller J, Helmer H, Husslein P, Pollak A, Spittler A, Förster-Waldl E (2013) Preterm neonates display altered plasmacytoid dendritic cell function and morphology. J Leukoc Biol 93(5):781–788
Zook EC, Kee BL (2016) Development of innate lymphoid cells. Nat Immunol 17(7):775–782
Kohl S (1999) Human neonatal natural killer cell cytotoxicity function. Pediatr Infect Dis J 18(7):635–637
Zlotoff DA, Schwarz BA, Bhandoola A (2008) The long road to the thymus: the generation, mobilization, and circulation of T-cell progenitors in mouse and man. Semin Immunopathol 30(4):371–382
Holt PG (2004) The role of genetic and environmental factors in the development of T-cell mediated allergic disease in early life. Paediatr Respir Rev 5(Suppl A):S27–S30
Marodi L (2002) Down-regulation of Th1 responses in human neonates. Clin Exp Immunol 128(1):1–2
Dowling DJ, Levy O (2014) Ontogeny of early life immunity. Trends Immunol 35(7):299–310
Simon AK, Hollander GA, McMichael A (2015) Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282(1821):20143085
Holt PG, Jones CA (2000) The development of the immune system during pregnancy and early life. Allergy 55:688–697
Walker JC, Smolders MAJC, Gemen EFA, Antonius TAJ, Leuvenink J, de Vries E (2011) Development of lymphocyte subpopulations in preterm infants. Scand J Immunol 73(1):53–58
Kobayashi RH, Hyman CJ, Stiehm ER (1980) Immunologic maturation in an infant born to a mother with agammaglobulinemia. Am J Dis Child 134(10):942–944
Ygberg S, Nilsson A (2012) The developing immune system - from foetus to toddler. Acta Paediatr 101(2):120–127
Tang ML, Kemp AS, Thorburn J, Hill DJ (1994) Reduced interferon-gamma secretion in neonates and subsequent atopy. The Lancet 344(8928):983–985
Warner JA, Jones CA, Jones AC, Warner JO (2000) Prenatal origins of allergic disease. J Allergy Clin Immunol 105:S493–S496
Strachan DP (1989) Hay fever, hygiene, and household size. BMJ 299(6710):1259–1260
Openshaw PJM, Yamaguchi Y, Tregoning JS (2004) Childhood infections, the developing immune system, and the origins of asthma. J Allergy Clin Immunol 114(6):1275–1277
Wills-Karp M, Santeliz J, Karp CL (2001) The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev Immunol 1(1):69–75
Sly PD, Boner AL, Björksten B, Bush A, Custovic A, Eigenmann PA, Gern JE, Gerritsen J, Hamelmann E, Helms PJ, Lemanske RF, Martinez F, Pedersen S, Renz H, Sampson H, von Mutius E, Wahn U, Holt PG (2008) Early identification of atopy in the prediction of persistent asthma in children. Lancet 372(9643):1100–1106
Jartti T, Smits HH, Bønnelykke K, Bircan O, Elenius V, Konradsen JR, Maggina P, Makrinioti H, Stokholm J, Hedlin G, Papadopoulos N, Ruszczynski M, Ryczaj K, Schaub B, Schwarze J, Skevaki C, Stenberg-Hammar K, Feleszko W, EAACI Task Force on Clinical Practice Recommendations on Preschool Wheeze (2019) Bronchiolitis needs a revisit: distinguishing between virus entities and their treatments. Allergy 74(1):40–52
Boraschi D, Italiani P (2018) Innate immune memory: time for adopting a correct terminology. Front Immunol 9:799
Krusche J, Twardziok M, Rehbach K, Böck A, Tsang MS, Schröder PC et al (2019) TNFAIP3 is a key player in childhood asthma development and environment-mediated protection. J Allergy Clin Immunol
Liu T, Valdez R, Yoon PW, Crocker D, Moonesinghe R, Khoury MJ (2009) The association between family history of asthma and the prevalence of asthma among US adults: National Health and Nutrition Examination Survey, 1999-2004. Genet Med 11(5):323–328
Paaso EMS, Jaakkola MS, Lajunen TK, Hugg TT, Jaakkola JJK (2013) The importance of family history in asthma during the first 27 years of life. Am J Respir Crit Care Med 188(5):624–626
Thomsen SF, Duffy DL, Kyvik KO, Backer V (2010) Genetic influence on the age at onset of asthma: a twin study. J Allergy Clin Immunol 126(3):626–630
Slager RE, Hawkins GA, Li X, Postma DS, Meyers DA, Bleecker ER (2012) Genetics of asthma susceptibility and severity. Clin Chest Med 33(3):431–443
Karmaus W, Arshad H, Mattes J (2001) Does the sibling effect have its origin in utero? Investigating birth order, cord blood immunoglobulin E concentration, and allergic sensitization at age 4 years. Am J Epidemiol 154(10):909–915
Devereux G, Barker RN, Seaton A (2002) Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy 32(1):43–50
Penders J, Gerhold K, Stobberingh EE, Thijs C, Zimmermann K, Lau S et al (2013) Establishment of the intestinal microbiota and its role for atopic dermatitis in early childhood. J Allergy Clin Immunol 132(3):601–607.e8
Strachan DP, Aït-Khaled N, Foliaki S, Mallol J, Odhiambo J, Pearce N, Williams HC, ISAAC Phase Three Study Group (2015) Siblings, asthma, rhinoconjunctivitis and eczema: a worldwide perspective from the International Study of Asthma and Allergies in Childhood. Clin Exp Allergy 45(1):126–136
Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, von Berg A, Bufe A, Rietschel E, Heinzmann A, Simma B, Frischer T, Willis-Owen SA, Wong KC, Illig T, Vogelberg C, Weiland SK, von Mutius E, Abecasis GR, Farrall M, Gut IG, Lathrop GM, Cookson WO (2007) Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 448(7152):470–473
James B, Milstien S, Spiegel S 2019 Ormdl3 and allergic asthma: from physiology to pathology. J Allergy Clin Immunol
Lluis A, Schedel M, Liu J, Illi S, Depner M, von Mutius E et al (2011) Asthma-associated polymorphisms in 17q21 influence cord blood ORMDL3 and GSDMA gene expression and IL-17 secretion. J Allergy Clin Immunol 127(6):1587–94.e6
Laubhahn K, Eckert J, Böck A, Hildebrand V, Unterschemmann S, Klucker E et al. Pathway analysis: genetic influences of 17q21 risk-polymorphisms and immune on subsequent childhood wheeze. unpublished.
Kim KW, Ober C (2019) Lessons learned from GWAS of asthma. Allergy Asthma Immunol Res 11(2):170–187
Bønnelykke K, Sleiman P, Nielsen K, Kreiner-Møller E, Mercader JM, Belgrave D, den Dekker H, Husby A, Sevelsted A, Faura-Tellez G, Mortensen LJ, Paternoster L, Flaaten R, Mølgaard A, Smart DE, Thomsen PF, Rasmussen MA, Bonàs-Guarch S, Holst C, Nohr EA, Yadav R, March ME, Blicher T, Lackie PM, Jaddoe VW, Simpson A, Holloway JW, Duijts L, Custovic A, Davies DE, Torrents D, Gupta R, Hollegaard MV, Hougaard DM, Hakonarson H, Bisgaard H (2014) A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet 46(1):51–55
Bouzigon E, Corda E, Aschard H, Dizier M-H, Boland A, Bousquet J, Chateigner N, Gormand F, Just J, le Moual N, Scheinmann P, Siroux V, Vervloet D, Zelenika D, Pin I, Kauffmann F, Lathrop M, Demenais F (2008) Effect of 17q21 variants and smoking exposure in early-onset asthma. N Engl J Med 359(19):1985–1994
Calışkan M, Bochkov YA, Kreiner-Møller E, Bønnelykke K, Stein MM, Du G et al (2013) Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N Engl J Med 368(15):1398–1407
Stokholm J, Chawes BL, Vissing N, Bønnelykke K, Bisgaard H (2018) Cat exposure in early life decreases asthma risk from the 17q21 high-risk variant. J Allergy Clin Immunol 141(5):1598–1606
Davidson EJ, Yang IV (2018) Role of epigenetics in the development of childhood asthma. Curr Opin Allergy Clin Immunol 18(2):132–138
Hill MR, James AL, Faux JA, Ryan G, Hopkin JM, Le Söuef P et al (1995) Fc epsilon RI-beta polymorphism and risk of atopy in a general population sample. BMJ 311(7008):776–779
Gao L, Millstein J, Siegmund KD, Dubeau L, Maguire R, Gilliland FD et al (2017) Epigenetic regulation of AXL and risk of childhood asthma symptoms. Clin Epigenetics 9:121
Arathimos R, Suderman M, Sharp GC, Burrows K, Granell R, Tilling K et al (2017) Epigenome-wide association study of asthma and wheeze in childhood and adolescence. Clin Epigenetics 9:112
Yang IV, Pedersen BS, Liu A, O’Connor GT, Teach SJ, Kattan M et al (2015) DNA methylation and childhood asthma in the inner city. J Allergy Clin Immunol 136(1):69–80
Jirtle RL, Skinner MK (2007) Environmental epigenomics and disease susceptibility. Nat Rev Genet 8(4):253–262
Sofer T, Baccarelli A, Cantone L, Coull B, Maity A, Lin X et al (2013) Exposure to airborne particulate matter is associated with methylation pattern in the asthma pathway. Epigenomics 5(2):147–154
Munthe-Kaas MC, Bertelsen RJ, Torjussen TM, Hjorthaug HS, Undlien DE, Lyle R, Gervin K, Granum B, Mowinckel P, Carlsen KH, Carlsen KC (2012) Pet keeping and tobacco exposure influence CD14 methylation in childhood. Pediatr Allergy Immunol 23(8):747–754
Schaub B, Liu J, Höppler S, Schleich I, Huehn J, Olek S et al (2009) Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol 123(4):774–82.e5
Ege MJ, Herzum I, Büchele G, Krauss-Etschmann S, Lauener RP, Roponen M et al (2008) Prenatal exposure to a farm environment modifies atopic sensitization at birth. J Allergy Clin Immunol 122(2):407-12–412.e1-4
Pfefferle PI, Büchele G, Blümer N, Roponen M, Ege MJ, Krauss-Etschmann S et al (2010) Cord blood cytokines are modulated by maternal farming activities and consumption of farm dairy products during pregnancy: the PASTURE Study. J Allergy Clin Immunol 125(1):108–15.e1-3
Korten I, Ramsey K, Latzin P (2017) Air pollution during pregnancy and lung development in the child. Paediatr Respir Rev 21:38–46
Hertz-Picciotto I, Herr CEW, Yap P-S, Dostál M, Shumway RH, Ashwood P et al (2005) Air pollution and lymphocyte phenotype proportions in cord blood. Environ Health Perspect 113(10):1391–1398
Latzin P, Frey U, Armann J, Kieninger E, Fuchs O, Röösli M, Schaub B (2011) Exposure to moderate air pollution during late pregnancy and cord blood cytokine secretion in healthy neonates. PLoS One 6(8):e23130
Strzelak A, Ratajczak A, Adamiec A, Feleszko W 2018 Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health; 15(5).
Nowak E, Neuner A, Landgraf-Rauf K, Schaub B (2017) Asthma und Allergieprävention. Pädiatrie Up2date 12(02):143–159
Krusche J, Schaub B (2018) Frühe Umweltexposition im Leben – Schutz oder Risiko für allergische Erkrankungen. AL 41(08):348–358
Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, Prescott SL (2003) Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol 112(6):1178–1184
Hornsby E, Pfeffer PE, Laranjo N, Cruikshank W, Tuzova M, Litonjua AA et al (2018) Vitamin D supplementation during pregnancy: effect on the neonatal immune system in a randomized controlled trial. J Allergy Clin Immunol 141(1):269–278.e1
van den Elsen LWJ, Garseen J, Burcelin R, Verhasselt V 2019 Shaping the gut microbiota by breastfeeding: the gateway to allergy prevention? Front Pediatr; 7(49)
Douros K, Moustaki M, Tsabouri S, Papadopoulou A, Papadopoulos M, Priftis KN (2017) Prenatal maternal stress and the risk of asthma in children. Front Pediatr 5(202)
Zazara DE, Perani CV, Solano ME, Arck PC (2018) Prenatal stress challenge impairs fetal lung development and asthma severity sex-specifically in mice. J Reprod Immunol 125:100–105
Hughes CH, Jones RC, Wright DE, Dobbs FF (1999) A retrospective study of the relationship between childhood asthma and respiratory infection during gestation. Clin Exp Allergy 29(10):1378–1381
Köksal BT, Ozbek OY, Bayraktar N, Yazici AC (2014) Evaluation of angiopoietin 1 and 2, vascular endothelial growth factor, and tumor necrosis factor alpha levels in asthmatic children. Allergy Asthma Proc 35(6):482–488
Zhao D, Su H, Cheng J, Wang X, Xie M, Li K, Wen L, Yang H (2015) Prenatal antibiotic use and risk of childhood wheeze/asthma: a meta-analysis. Pediatr Allergy Immunol 26(8):756–764
Bai L, Zhao D, Cheng Q, Zhang Y, Wang S, Zhang H, Xie M, He R, Su H (2019) Trimester-specific association between antibiotics exposure during pregnancy and childhood asthma or wheeze: the role of confounding. Ann Epidemiol 30:1–8
Oyama N, Sudo N, Sogawa H, Kubo C (2001) Antibiotic use during infancy promotes a shift in the T(H)1/T(H)2 balance toward T(H)2-dominant immunity in mice. J Allergy Clin Immunol 107(1):153–159
Tollånes MC, Moster D, Daltveit AK, Irgens LM (2008) Cesarean section and risk of severe childhood asthma: a population-based cohort study. J Pediatr 153(1):112–116
McKeever TM, Lewis SA, Smith C, Hubbard R (2002) Mode of delivery and risk of developing allergic disease. J Allergy Clin Immunol 109(5):800–802
Huang L, Chen Q, Zhao Y, Wang W, Fang F, Bao Y (2015) Is elective cesarean section associated with a higher risk of asthma? A meta-analysis. J Asthma 52(1):16–25
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R (2010) Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 107(26):11971–11975
Werner A, Ramlau-Hansen CH, Jeppesen SK, Thulstrup AM, Olsen J (2007) Caesarean delivery and risk of developing asthma in the offspring. Acta Paediatr 96(4):595–596
Källén B, Finnström O, Nygren K-G, Otterblad OP (2013) Association between preterm birth and intrauterine growth retardation and child asthma. Eur Respir J 41(3):671–676
von Mutius E, Vercelli D (2010) Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 10(12):861–868
Kirjavainen PV, Karvonen AM, Adams RI, Täubel M, Roponen M, Tuoresmäki P, Loss G, Jayaprakash B, Depner M, Ege MJ, Renz H, Pfefferle PI, Schaub B, Lauener R, Hyvärinen A, Knight R, Heederik DJJ, von Mutius E, Pekkanen J (2019) Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat Med 25(7):1089–1095
Braun-Fahrländer C, Lauener R (2003) Farming and protective agents against allergy and asthma. Clin Exp Allergy 33(4):409–411
Lluis A, Depner M, Gaugler B, Saas P, Casaca VI, Raedler D, Michel S, Tost J, Liu J, Genuneit J, Pfefferle P, Roponen M, Weber J, Braun-Fahrländer C, Riedler J, Lauener R, Vuitton DA, Dalphin JC, Pekkanen J, von Mutius E, Schaub B, Protection Against Allergy: Study in Rural Environments Study Group (2014) Increased regulatory T-cell numbers are associated with farm milk exposure and lower atopic sensitization and asthma in childhood. J Allergy Clin Immunol 133(2):551–559
Kääriö H, Nieminen JK, Karvonen AM, Huttunen K, Schröder PC, Vaarala O, von Mutius E, Pfefferle PI, Schaub B, Pekkanen J, Hirvonen MR, Roponen M (2016) Circulating dendritic cells, farm exposure and asthma at early age. Scand J Immunol 83(1):18–25
Schröder PC, Li J, Wong GWK, Schaub B (2015) The rural-urban enigma of allergy: what can we learn from studies around the world? Pediatr Allergy Immunol 26(2):95–102
Holbreich M, Genuneit J, Weber J, Braun-Fahrländer C, Waser M, von Mutius E (2012) Amish children living in northern Indiana have a very low prevalence of allergic sensitization. J Allergy Clin Immunol 129(6):1671–1673
Motika CA, Papachristou C, Abney M, Lester LA, Ober C (2011) Rising prevalence of asthma is sex-specific in a US farming population. J Allergy Clin Immunol 128(4):774–779
Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, Ledford JG, Marques Dos Santos M, Anderson RL, Metwali N, Neilson JW, Maier RM, Gilbert JA, Holbreich M, Thorne PS, Martinez FD, von Mutius E, Vercelli D, Ober C, Sperling AI (2016) Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med 375(5):411–421
Feng M, Yang Z, Pan L, Lai X, Xian M, Huang X, Chen Y, Schröder PC, Roponen M, Schaub B, Wong GW, Li J (2016) Associations of early life exposures and environmental factors with asthma among children in rural and urban areas of Guangdong. China Chest 149(4):1030–1041
Martikainen M-V, Kääriö H, Karvonen A, Schröder PC, Renz H, Kaulek V, Dalphin JC, von Mutius E, Schaub B, Pekkanen J, Hirvonen MR, Roponen M (2015) Farm exposures are associated with lower percentage of circulating myeloid dendritic cell subtype 2 at age 6. Allergy 70(10):1278–1287
Bowatte G, Lodge CJ, Knibbs LD, Lowe AJ, Erbas B, Dennekamp M et al (2017) Traffic-related air pollution exposure is associated with allergic sensitization, asthma, and poor lung function in middle age. J Allergy Clin Immunol 139(1):122–129.e1
Perez L, Declercq C, Iñiguez C, Aguilera I, Badaloni C, Ballester F, Bouland C, Chanel O, Cirarda FB, Forastiere F, Forsberg B, Haluza D, Hedlund B, Cambra K, Lacasaña M, Moshammer H, Otorepec P, Rodríguez-Barranco M, Medina S, Künzli N (2013) Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network). Eur Respir J 42(3):594–605
Zuo L, Otenbaker NP, Rose BA, Salisbury KS (2013) Molecular mechanisms of reactive oxygen species-related pulmonary inflammation and asthma. Mol Immunol 56(1-2):57–63
Fernvik E, Scharnweber T, Knopp D, Niessner R, Vargaftig BB, Peltre G (2002) Effects of fractions of traffic particulate matter on TH2-cytokines, IgE levels, and bronchial hyperresponsiveness in mice. J Toxicol Environ Health Part A 65(15):1025–1045
van Dyken SJ, Locksley RM (2018) Chitins and chitinase activity in airway diseases. J Allergy Clin Immunol 142(2):364–369
Zhang Z, Biagini Myers JM, Brandt EB, Ryan PH, Lindsey M, Mintz-Cole RA et al (2017) β-Glucan exacerbates allergic asthma independent of fungal sensitization and promotes steroid-resistant TH2/TH17 responses. J Allergy Clin Immunol 139(1):54–65.e8
Ihuoma H, Belgrave DC, Murray CS, Foden P, Simpson A, Custovic A (2018) Cat ownership, cat allergen exposure, and trajectories of sensitization and asthma throughout childhood. J Allergy Clin Immunol 141(2):820–822.e7
Hesselmar B, Hicke-Roberts A, Lundell A-C, Adlerberth I, Rudin A, Saalman R, Wennergren G, Wold AE (2018) Pet-keeping in early life reduces the risk of allergy in a dose-dependent fashion. PLoS One 13(12):e0208472
Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL et al (2010) Man’s best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol 126(2):410-2–412.e1-3
World Health Organization. World Health Organization recommendations on postnatal care of the mother and newborn. 2013.
Muraro A, Halken S, Arshad SH, Beyer K, Dubois AEJ, Du Toit G et al (2014) EAACI food allergy and anaphylaxis guidelines. Primary prevention of food allergy. Allergy 69(5):590–601
Miliku K, Azad MB (2018) Breastfeeding and the Developmental Origins of Asthma: Current Evidence, Possible Mechanisms, and Future Research Priorities. Nutrients:10(8)
Labbok MH, Clark D, Goldman AS (2004) Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol 4(7):565–572
Adlerberth I, Wold AE (2009) Establishment of the gut microbiota in western infants. Acta Paediatr 98(2):229–238
Oddy WH, de Klerk NH, Sly PD, Holt PG (2002) The effects of respiratory infections, atopy, and breastfeeding on childhood asthma. Eur Respir J 19(5):899–905
Mazzocchi A, Venter C, Maslin K (2017) Agostoni C. The role of nutritional aspects in food allergy: prevention and management. Nutrients 9(8)
Edwards MR, Walton RP, Jackson DJ, Feleszko W, Skevaki C, Jartti T et al (2018) The potential of anti-infectives and immunomodulators as therapies for asthma and asthma exacerbations. Allergy 73(1):50–63
Murrison LB, Brandt EB, Myers JB, Hershey GKK (2019) Environmental exposures and mechanisms in allergy and asthma development. J Clin Invest 129(4):1504–1515
Heymann PW, Nguyen H-T, Steinke JW, Turner RB, Woodfolk JA, Platts-Mills TAE et al (2017) Rhinovirus infection results in stronger and more persistent genomic dysregulation: evidence for altered innate immune response in asthmatics at baseline, early in infection, and during convalescence. PLoS One 12(5):e0178096
Fenoy IM, Chiurazzi R, Sánchez VR, Argenziano MA, Soto A, Picchio MS et al (2012) Toxoplasma gondii infection induces suppression in a mouse model of allergic airway inflammation. PLoS One 7(8):e43420
Amedei A, Codolo G, Del Prete G, de Bernard M, D’Elios MM (2010) The effect of Helicobacter pylori on asthma and allergy. J Asthma Allergy 3:139–147
Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M (2010) Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep 10(1):3–12
Kelly D, King T, Aminov R (2007) Importance of microbial colonization of the gut in early life to the development of immunity. Mutat Res 622(1-2):58–69
Arrieta M-C, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S et al (2015) Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7(307):307ra152
Stokholm J, Blaser MJ, Thorsen J, Rasmussen MA, Waage J, Vinding RK, Schoos AM, Kunøe A, Fink NR, Chawes BL, Bønnelykke K, Brejnrod AD, Mortensen MS, al-Soud WA, Sørensen SJ, Bisgaard H (2018) Maturation of the gut microbiome and risk of asthma in childhood. Nat Commun 9(1):141
McAleer JP, Kolls JK (2018) Contributions of the intestinal microbiome in lung immunity. Eur J Immunol 48(1):39–49
Birzele LT, Depner M, Ege MJ, Engel M, Kublik S, Bernau C, Loss GJ, Genuneit J, Horak E, Schloter M, Braun-Fahrländer C, Danielewicz H, Heederik D, von Mutius E, Legatzki A (2017) Environmental and mucosal microbiota and their role in childhood asthma. Allergy 72(1):109–119
Depner M, Ege MJ, Cox MJ, Dwyer S, Walker AW, Birzele LT et al (2017) Bacterial microbiota of the upper respiratory tract and childhood asthma. J Allergy Clin Immunol 139(3):826–834.e13
Du Toit G, Tsakok T, Lack S, Lack G (2016) Prevention of food allergy. J Allergy Clin Immunol 137(4):998–1010
Nakamura T, Kloetzer WS, Brams P, Hariharan K, Chamat S, Cao X, LaBarre M, Chinn PC, Morena RA, Shestowsky WS, Li YP, Chen A, Reff ME (2000) In vitro IgE inhibition in B cells by anti-CD23 monoclonal antibodies is functionally dependent on the immunoglobulin Fc domain. Int J Immunopharmacol 22(2):131–141
Zhang W, Lin C, Sampath V, Nadeau K (2018) Impact of allergen immunotherapy in allergic asthma. Immunotherapy 10(7):579–593
Peters SP, Busse WW (2017) New and anticipated therapies for severe asthma. J Allergy Clin Immunol Pract 5(5S):S15–S24
Casale TB (2017) Biologics and biomarkers for asthma, urticaria, and nasal polyposis. J Allergy Clin Immunol 139(5):1411–1421
Funding
This work was supported by the DFG grants DFG-SCHA 997/8-1; DFG-SCHA 997/7-1 as well as BMBF GILKUJ-39 and FöFoLe 52/2018.
Author information
Authors and Affiliations
Contributions
JK, SB, and BS conceived the study. JK and SB drafted the manuscript; all authors have revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no conflicts of interest to declare.
Additional information
This article is a contribution to the special issue on Asthma: Novel developments from bench to bedside - Guest Editor: Bianca Schaub
Literature search strategy
We searched PubMed for articles published in English until 08.08.2019.
We identified relevant articles published before 2000 through a search via Google Scholar and through reviewing the articles and their relevant references cited. This was by no means an exhaustive search.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Johanna Krusche and Sarah Basse both shared first authorship.
Rights and permissions
About this article
Cite this article
Krusche, J., Basse, S. & Schaub, B. Role of early life immune regulation in asthma development. Semin Immunopathol 42, 29–42 (2020). https://doi.org/10.1007/s00281-019-00774-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-019-00774-z