Abstract
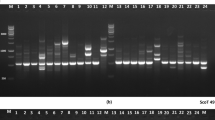
In this study, we analysed the impact of population size and isolation on the genetic variation of the short-lived alpine plant species Gentianella campestris ssp. campestris from two study regions (Allgäu and Karwendel) in the Northern calcareous Alps in Germany. We determined the size and isolation of the study populations and analysed genetic variation using amplified fragment length polymorphisms. Genetic variation of G. campestris ssp. campestris differed significantly between the two study regions. Genetic variation did not depend on population size. However, the level of genetic variation within populations was about three times lower in the Karwendel, where the species is much more isolated than in the Allgäu. Conversely, genetic variation among populations was much stronger in the Karwendel than in the Allgäu. Our results support the observation that the level of genetic variation within populations of alpine plant species may not only be affected by population size, but also by population isolation. Depending on the distance among populations, gene flow by exchange of pollen and seeds triggers the influx of genetic variation, thereby sometimes superimposing the effects of population size. Our results suggest that for seed collections in conservation projects, not only population size, but also isolation should be considered.



Similar content being viewed by others
References
Ægisdóttir HH, Kuss P, Stöcklin J (2009) Isolated populations of a rare alpine plant show high genetic diversity and considerable population differentiation. Ann Bot 104:1313–1322
Aeschimann D, Lauber K, Moser DM, Theurillat JP (2004) Flora alpina, vol 2. Haupt, Bern
Beatty GE, McEvoy PM, Sweeney O, Provan J (2008) Range-edge effects promote clonal growth in peripheral populations of the one-sided wintergreen Orthilia secunda. Divers Distrib 14:546–555
BfN (2018) FloraWeb—Daten und Informationen zu Wildpflanzen und zur Vegetation Deutschlands. http://www.floraweb.de/. Accessed 15 Dec 2017
Bonin A, Belleman E, Eidesen PB, Pompanon F, Brochmann C, Taberlet P (2004) How to track and assess genotyping errors in population genetic studies. Mol Ecol 13:3261–3273
Brütting C, Meyer S, Kühne P, Hensen I, Wesche K (2012) Spatial genetic structure and low diversity of the rare arable plant Bupleurum rotundifolium L. indicate fragmentation in Central Europe. Agric Ecosyst Environ 161:70–77
Busch V, Reisch C (2016) Population size and land use affect the genetic variation and performance of the endangered plant species Dianthus seguieri ssp. glaber. Conserv Genet 17:425–436. https://doi.org/10.1007/s10592-015-0794-1
Bylebyl K, Poschlod P, Reisch C (2008) Genetic variation of Eryngium campestre L. (Apiaceae) in Central Europe. Mol Ecol 17:3379–3388
Cruzan M (2001) Population size and fragmentation thresholds for the maintenance of genetic diversity in the herbaceaous endemic Scutellaria montana (Lamiaceae). Evolution 55:1569–1580
Dorken ME, Eckert CG (2001) Severely reduced sexual reproduction in northern populations of a clonal plant, Decodon verticillatus (Lythraceae). J Ecol 89:339–350
Durka W (1999) Genetic diversity in peripheral and subcentral populations of Corrigiola litoralis L. (Illecebraceae). Heredity 83:476–484
Durka W et al (2017) Genetic differentiation within multiple common grassland plants supports seed transfer zones for ecological restoration. J Appl Ecol 54:116–126
Earl DA, Vonholdt BM (2012) STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour 4:359–361. https://doi.org/10.1007/s12686-011-9548-7
Eckert CG, Samis E, Lougheed SC (2008) Genetic variation across species’ geographical ranges: the central marginal hypothesis and beyond. Mol Ecol 17:1170–1188
Eckstein RL, O’Neill RA, Danihelka J, Otte A, Köhler W (2006) Genetic structure among and within peripheral and central populations of three endangered floodplain violets. Mol Ecol 15:2367–2379
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software structure: a simulation studie. Mol Ecol 14:2611–2620
Evans MEK, Dolan RW, Menges ES, Gordon D (2000) Genetic diversity and reproductive biology in Warea carteri (Brassicaceae), a narrowly endemic Florida scrub annual. Am J Bot 87:372–381
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Falush D, Stephens M, Pritchard JK (2007) Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol Ecol Notes 7:574–578. https://doi.org/10.1111/j.1471-8286.2007.01758.x
Fischer M, Matthies D (1998) RAPD variation in relation to population size and plant fitness in the rare Gentianella germanica (Gentianaceae). Am J Bot 85:811–819
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University Press, Cambridge
Gabel A-R, Sattler J, Reisch C (2017) Genetic variation and performance of the alpine plant species Dianthus callizonus differ in two elevational zones of the. Carpathians Alpine Bot 127:65–74
García D, Zamora R, Gómez JM, Jordano P, Hódar JA (2000) Geographical variation in seed production, predation and abortion in Juniperus communis throughout its range in Europe. J Ecol 88:436–446
Greene SL, Kisha TJ, Yu L-X, Parra-Quijano M (2014) Conserving plants in gene banks and nature: investigating complementarity with Trifolium thompsonii morton. PLoS One. https://doi.org/10.1371/journal.pone.0105145
Greimler J, Dobes C (2000) High genetic differentiation in relict lowland populations of Gentianella austriaca (A and J Kern) Holub (Gentianaceae). Plant Biol 2:628–637
Hamilton JA, Eckert CG (2007) Population genetic consequences of geographic disjunction: a prairie plant isolated on Great Lakes alvars. Mol Ecol 16:1649–1660
Heelemann S, Krug CB, Esler KJ, Poschlod P, Reisch C (2014) Low impact of fragmentation on genetic variation within and between remnant populations of the typical renosterveld species Nemesia barbata in South Africa. Biochem Syst Ecol 54:59–64
Hensen I, Kilian C, Wagner V, Durka W, Pusch J, Wesche K (2010) Low genetic variability and strong differentiation among isolated populations of the rare steppe grass Stipa capillata L. in Central Europe. Plant Biol 12:526–536
Honnay O, Jacquemyn H (2007) Susceptibility of common and rare species to the genetic consequences of habitat fragmentation. Conserv Biol 21:823–831
Huhta A-P, Lennartsson T, Tuomi J, Rautio P, Laine K (2000) Tolerance of Gentianella campestris in relation to damage intensity: an interplay between apical dominance and herbivory. Evol Ecol 14:373–392
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267. https://doi.org/10.1093/molbev/msj030
Jacquemyn H, Vandepitte K, Roldán-Ruiz I, Honnay O (2009) Rapid loss of genetic variation in a founding population of Primula elatior (Primulaceae) after colonization. Ann Bot 103:777–783
Jump AS, Woodward FI (2003) Seed production and population density decline approaching the range-edge of Cirsium species. N Phytol 160:349–358
Kaulfuß F, Reisch C (2017) Reintroduction of the endangered and endemic plant species Cochlearia bavarica—implications from conservation genetics. Ecol Evol 7:11100–11112
Königer J, Rebernig CA, Brabec J, Kiehl K, Greimler J (2012) Spatial and temporal determinants of genetic structure in Gentianella bohemica. Ecol Evol 2:363–368
Kuss P, Pluess AR, Ǽgisdóttir HH, Stöcklin J (2008) Spatial isolation and genetic differentiation in naturally fragmented plant populations of the Swiss Alps. J Plant Ecol 1:149–159
Lammi A, Siikamäki P, Mustajärvi K (1999) Genetic diversity, population size, and fitness in central and peripheral populations of a rare plant Lychnis viscaria. Conserv Biol 13:1069–1078
Lande R (1988) Genetics and demography in biological conservation. Science 241:1455–1460
Lawton JH (1993) Range, population abundance and conservation. Trends Ecol Evol 8:409–413
Leimu R, Mutikainen P, Koricheva J, Fischer M (2006) How general are positive relationships between plant population size, fitness and genetic variation. J Ecol 94:942–952
Lennartsson T, Tuomi J, Nilsson P (1997) Evidence for an evolutionary history of overcompensation in the grassland biennial Gentianella campestris (Gentianaceae). Am Nat 149:1147–1155
Lennartsson T, Oostermeijer JGB, Van Dijk J, Den Nijs HCM (2000) Ecological significance of floral reproductive traits in Gentianella campestris (Gentianaceae). Basic Appl Ecol 1:69–81
Luikart G, Ryman N, Tallmon DA, Schwartz MK, Allendorf FW (2010) Estimation of census and effective population sizes: the increasding usefulness of DNA-based approaches. Conserv Genet 11:355–373
Lynch M (1991) The genetic interpretation of inbreeding depression and outbreeding depression. Evolution 45:622–629
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Matthies D, Bräuer I, Maiboom W, Tscharntke T (2004) Population size and the risk of extinction: empirical evidence from rare plants. Oikos 105:481–488
Oostermeijer JGB (1996) Population size, genetic variation, and related parameters in small, isolated plant populations: a case study. In: Settele J, Margules CR, Poschlod P, Henle K (eds) Species survival in fragmented landscapes. Kluwer Academic, Dordrecht, pp 61–68
Ouborg NJ, Vergeer P, Mix C (2006) The rough edges of the conservation genetics paradigm. J Ecol 94:1233–1248
Paun O, Schönswetter P, Winkler M, IntraBioDiv-Consortium, Tribsch A (2008) Historical divergence vs. contemporary gene flow: evolutionary history of the calcicole Ranunculus alpestris group (Ranunculaceae) in the European Alps and the Carpathians. Mol Ecol 17:4263–4275
Peakall R, Smouse PE (2006) GENALEX 6: genetic analyses in Excel. Population genetic software for teaching and reseach. Mol Ecol Notes 6:288–295
Peterson A, Bartish IV, Peterson J (2008) Effects of population size on genetic diversity, fitness and pollinator community composition in fragmented populations of Anthericum liliago L. Plant Ecol 198:101–110
Pfeifer M, Schatz B, Picó FX, Passalacqua NG, Fay MF, Carey PD, Jeltsch F (2009) Phylogeography and genetic structure of the orchid Himanthoglossum hircinum (L.) Spreng. across its European central-marginal gradient. J Biogeogr 36:2353–2365
Plenk K, Göd F, Kriechbaum M, Kropf M (2016) Genetic and reproductive characterisation of seasonal flowering morphs of Gentianella bohemica revealed strong reproductive isolation and possible single origin. Plant Biol 18:111–123
Pluess AR, Stöcklin J (2004) Genetic diversity and fitness in Scabiosa columbaria in the Swiss Jura in relation to population size. Conserv Genet 5:145–156
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Pritchard JK, Wen X, Falush D (2007) Documentation for structure software: version 2.2
Raabova J, Van Rossum F, Jacquemart AL, Raspe O (2015) Population size affects genetic diversity and fine-scale spatial genetic structure in the clonal distylous herb Menyanthes trifoliata perspectives. Plant Ecol Evol Syst 17:193–200. https://doi.org/10.1016/j.ppees.2015.02.005
R-Core-Team (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing,, Vienna, Austria, http://www.R-project.org/. Accessed 23 Apr 2018
Reed DH, Frankham R (2003) Corelation between fitness and genetic diversity. Conserv Biol 17:230–237
Reisch C (2007) Genetic structure of Saxifraga tridactylites (Saxifragaceae) from natural and man-made habitats. Conserv Genet 8:893–902
Reisch C (2008) Glacial history of Saxifraga paniculata (Saxifragaceae)—molecular biogeography of a disjunct arctic-alpine species in Europe and North America. Biol J Linn Soc 93:385–398
Reisch C, Bernhardt-Römermann M (2014) The impact of study design and life history traits on genetic variation of plants determined with AFLPs. Plant Ecol 215:1493–1511
Reisch C, Poschlod P, Wingender R (2003) Genetic variation of Saxifraga paniculata Mill. (Saxifragaceae): molecular evidence for glacial relict endemism in central Europe. Biol J Linn Soc 80:11–21
Reisch C, Schmid C, Hartig F (2018) A comparison of methods for estimating plant population size. Biodivers Conserv 27:2021–2028
Rogers SO, Bendich AJ (1994) Extraction of total cellular DNA from plants, algae and fungi. In: Gelvin SB, Schilperoort RA (eds) Plant molecular biology manual, 2 edn. Kluwer Academic, Dordrecht, pp 1–8
Sagarin RD, Gaines SD (2002) The ‘abundant centre’ distribution: to what extent is it a biogeographical rule? Ecol Lett 5:137–147
Sagarin RD, Gaines SD, Gaylord B (2006) Moving beyond assumptions to understand abundance distributions across the ranges of species. Trend Ecol Evol 21:524–530
Schönfelder P, Bresinsky A (eds) (1990) Verbreitungsatlas der Farn- und Blütenpflanzen Bayerns. Ulmer, Stuttgart
Sebald O, Seybold S, Philippi G, Wörz A (1998) Farn- und Blütenpflanzen Baden-Württembergs, vol 7. Ulmer, Stuttgart
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236:787–792
Steffan-Dewenter I, Tscharntke T (1999) Effects of habitat isolation on pollinator communities and sed set. Oecologia 12:432–440
Tausch S, Leipold M, Reisch C, Poschlod P (2015) Genbank Bayern Arche—a contribution to the permanent conservation of threatened plants in. Bavaria Anliegen Nat 37:82–91
Vekemans X (2002) AFLP-surv version 1.0 vol 16. Distributed by the author. Laboratoire de Génétique et Ecologie Végétale. Université Libre de Bruxelles, Bruxelles, Belgium
Vogler F, Reisch C (2013) Vital survivors: low genetic variation but high germination in glacial relict populations of the typical rock plant Draba aizoides. Biodivers Conserv 22:1301–1316
Wang J, Santiago E, Caballero A (2016) Prediction and estimation of effective population size. Heredity 117:193–206
Whitlock R, Hipperson H, Thompson DBA, Butlin RK, Burke T (2016) Consequences of in-situ strategies for the conservation of plant genetic diversity. Biol Conserv 203:134–142
Young A, Boyle T, Brown T (1996) The population genetic consequences of habitat fragmentation for plants. Trend Ecol Evol 11:413–418
Acknowledgements
We would like to thank Veronika Bäuerlein and Petra Schitko for lab work, Sabine Fischer for her help with the map, Peter Poschlod for lively discussions and the government of the Upper Palatinate for financial support.
Author information
Authors and Affiliations
Contributions
CR conceived and designed the study. Both authors contributed to data analysis. CR wrote the first draft of the manuscript, BH contributed to revisions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest in relation with this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reisch, C., Hoiß, B. Genetic variation of Gentianella campestris ssp. campestris in the Northern Alps: how important are population size and isolation?. Alp Botany 129, 11–20 (2019). https://doi.org/10.1007/s00035-019-00216-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00035-019-00216-4