Abstract
The presented article shows the studies of hydrocracking process of fuel oil with the purpose of obtaining light oil products (benzene and diesel fractions) from heavy oil residues (fuel oil), thus, deepening the refining of oil. The hydrocracking of fuel oil was conducted in the presence of halloysite modified with transition metals (Mo, Ni). Toward this end, halloysite was modified by two different methods—absorption and ion-exchange methods. It was shown that, at optimal conditions (430 °C, 4 MPa), 46.6% (wt.), 53.0% (wt.), 63.0% (wt.) and 83.0% (wt.) light oil products are obtained by the hydrocracking process of fuel oil carried out without catalyst, in the presence of unmodified halloysite, halloysite modified by absorption method and halloysite modified by ion-exchange method, respectively. The obtained benzene and diesel fractions after hydrorefining process can be added to fuels as components.
Similar content being viewed by others
Introduction
One of the important issues is to increase the refining depth of petroleum by developing high-quality, ecologically clean processes and technologies required for the modern progress level of the petrochemical industry. Hydrocracking process is one of the effective processes for obtaining motor fuels of high quality from heavy oil residues (fuel oil, goudron). Despite the numerous works of leading foreign companies in this field, up to the present time, a simple and efficient technology that allows to directly and efficiently process residual oil products to produce fuel fractions has not been created [1,2,3]. Taking this into account, intensive works are being done to improve the hydrocracking technology, in particular, to use reactors with a fluidized bed or a suspended single-use catalyst bed. In industry, a number of processes were mastered for the rundown processing of heavy hydrocarbon feedstock into distillate products under hydrogen pressure (15–27 MPa) [4].
Thus, H-Oil and LC-Fining processes were developed by Hydrocarbon Research (USA) and Lummus (USA) firms, respectively, and these processes were carried out on a fluidized bed of catalyst, for processing heavy raw materials into distillate products under hydrogen pressure 16–25 MPa [5, 6].
Ascending gas-feed stream provides maintenance of “fluidized” catalyst bed. In such a system, a uniform distribution of gas, liquid and catalyst over the cross-section of the reactor and the isothermal nature of the process was achieved. The process can be carried out with a moderate (65%) or high (85% and higher) degree of goudron conversion (overpoint 524 °C). In the latter case, the circulation of the vacuum residue is applied.
Industrial plants for this and other similar methods are used in Japan, Germany, Kuwait and other countries [5, 6].
In Japan, the process of thermal cracking was developed at a relatively low temperature in a fluidized bed of inert porous material. During the processing of heavy raw materials (with high metal content), a large yield of middle distillates and relatively low yield of gas and coke were achieved. Heavy metals were collected in the pores of an inert material. The process is developed in two versions: with and without hydrogen [7].
Because of great amount of heteroatomic compounds, heavy organic metal compounds and asphaltenes inside the fractions obtained from petroleum, the coke and metal-organic compounds precipitate on the surface of catalyst and that makes serious problems in refining. Simultaneously, the size of the high-molecular compounds undergoing hydrocracking may be bigger than the size of micro- and macropores of stationary catalysts in the form of tablets and granules. Besides, the process is always accompanied by irreversible precipitation of coke and metal compounds on the surface of catalyst. Therefore, it is significantly important to develop new progressive technology by using sterically accessible and constantly renewing the surface catalysts for deep refining of the residual products obtained from petroleum.
The selection of the catalysts for hydrocracking process is one of the main problems. In literature, for the catalysts of hydrocracking process, the following acidic components are most commonly used: amorphous aluminosilicates, Y-type zeolite, or their combination [8].
In some research studies [9], beta-zeolite catalysts were used in hydrocracking of heavy oil residues. In the catalysts applied for hydrocracking process, platinum or palladium was used as a hydrogenating component, because hydrogen is soluble in platinum and palladium and accelerates hydrogenation process by passing into an atomic state. Usually, nickel, cobalt, molybdenum, tungsten, or their combinations are used, too. The reason is that these metals are relatively cheaper than platinum and palladium. Also, when applying the listed above metals, catalysts are not contaminated with pollutants. These metals are added to carriers using impregnation, ion-exchange, precipitation, and co-precipitation methods. Depending on the used metal, the amount of impregnated metal is limited as follows: Pt (Pd) 0.1–3 wt%; Ni (Co) 2.5–5 wt%; Mo (W) 5–15 wt% in the form of sulfides.
The catalysts proposed by the authors [10] were investigated in the hydrocracking of the heavy oil residues (which contain 3.39% sulfur inside). It was suggested that the nature of the hydrogenating component (NiMo or NiW) weakly impacts the conversion of heavy oil residue and the yield of diesel fraction, but affects the properties of the obtained diesel fraction. In comparison with NiW/Al2O3-amorphous aluminosilicates, the NiMo/Al2O3-amorphous aluminosilicates catalyst ensures lower sulfur amount in the obtained diesel fraction.
In the literature [11], sulfides of transition metals on the carrier were used as a catalyst. The catalyst’s cracking function is usually explained by the presence of acid centers in aluminum oxide or aluminosilicate carriers. The properties of the catalyst of the hydrocracking process are determined by the ratio of hydrogenation centers on metal sulfide and the concentration of acids in the carrier. In relatively low amounts of hydrogenation centers, secondary processes can occur which lead to the production of light hydrocarbons, also to oligomerization and generation of coke in the acid centers. The deficit of the hydrogenation function of hydrocracking catalyst results in a rapid decrease in its activity. At the same time, high hydrogenation activity prevents the cracking process and the isomerization process prevails.
Based on the above background, the present study was dedicated to investigation of hydrocracking process of fuel oil obtained from Baku petroleum, in the presence of nanostructured halloysite.
Catalyst characterization
Halloysite is a two-layer aluminosilicate, mainly tubular in submicron diapason and chemically similar to kaolin. Halloysite is mined from natural deposits in USA, New Zealand, Korea, China, Turkey and other countries. These minerals formed from kaolinite as a result of influence of the atmospheric condition and hydrothermal processes, which are occured during the millions of years. Layers are twisted to nanotubes because of the deformation formed in the result of incompatibility of cages between aluminum oxide layers with silica anhydride placed close to each other [12,13,14,15].
Due to the nanotubular structure, halloysite particles potentially can be used in some fields of nanotechnology. These multi-layer tubes are usually used for capsulation of plastic composites, chemically and biologically active substances. Moreover, they can be covered with metals for obtaining conductive additives. Due to its porous structure and high catalytic activity, halloysite particles can be used in mines for cleaning water drain systems, in oil refining, and at the same time separating liquid and gaseous mixtures [13, 14, 16, 17].
Figure 1 shows the schematic view of halloysite (7 Å) nanotubes.
The outer diameter of tubes is 70 nm, on average it changes from 40 nm up to 100 nm. The inner lumen (hole) diameter changes between 10 and 50 nm and on average is equal to 20 nm. The length of tubes is in diapason from 0.5 to 20 μm [15].
Aluminosilicates are still intensively used as catalysts in different refining processes of petroleum hydrocarbons from the early development stages of the oil industry. There are several patents of USA on use of halloysites as a catalyst in the isomerization and cracking processes of hydrocarbons [18, 19]. In comparison with the other aluminosilicates, halloysite belongs to kaolinite clay minerals class with high Al/Si ratio. Availability of a great amount of acid centers in aluminum oxide and nanoparticles causes cracking of hydrocarbons. These acid centers accelerate heterolytic breaking of the chemical bonds, and this causes formation of unstable carbocations. The carbocations undergo decomposition of C–C bonds by re-grouping of chains and beta-elimination or hydride ion transmission. All these processes cause formation of radicals and ions with reacting capacity accelerating the cracking process [20].
The initial works on using halloysite nanotubes as a catalyst for hydrocarbon processing date back to the 1950s [21]. V. Offot and A. Vytaker from the company “Gulf R&D” obtained a catalyst on the basis of magnesium oxide and halloysite mixture for the conversion of the hydrocarbons. This catalyst had low coke formation in comparison with montmorillonite and other active cracking catalysts used at that time. Another advantage of halloysite was the higher reaction ability in cracking of hydrocarbons having a high amount of sulfur [21]. Since those years, the catalyst on the basis of halloysite attracted the attention of many researchers. D. Thentilli from the company “CHEVRON” has patented the catalyst on the basis of halloysite mixed with the metal oxides, especially Al, Mg, Si, Ti, B and Zr oxides for hydrorefining and hydrometalling of asphaltene-containing petroleum products (crude oil, shale oils, etc.) [19]. The oxides of Cr, V, Fe, Mo and other transition metals were also added to improve the performance of catalyst. It is shown that halloysite nanotubes with pore size of 20–70 nm interval are especially good for deasphalting of hydrocarbons. The deasphalting occurs faster with pure halloysite and mixture of halloysite with 5% aluminum oxide, and this shows a higher adsorption ability of the given porous substances. Transition of adsorbed hydrocarbons to another shape (hydrocracking, desulphurization, etc.) occurs in the presence of the oxides of the transition metals spread on halloysite [19].
Experimental section
For producing of fuel components from hydrocracking of fuel oil (which physico-chemical properties were given at Table 1), investigations were conducted at low pressure (0.5–6 MPa) in the presence of halloysite modified with transition metals (Mo,Ni) by the method of deposition from nanostructured halloysite and gas phase for obtaining additional light oil products with the purpose of deepening oil refining depth.
The preparation process of catalytic systems from nanostructured natural halloysite mineral containing metal in its composition for realization of fuel oil hydrocracking is implemented by two methods: absorption and ion-exchange methods.
- 1.
Absorption method The initial carrier of the catalyst complex—γ-Al2O3 for synthesis of Mo, Ni/Al2O3 used for preparation of halloysite (Mo, Ni) catalyst was obtained on the basis of hydrolysis of 1,2 dichloroethane in the presence of aluminum metal. C7–C14 liquid paraffin hydrocarbons were used as the main solvent and the process temperature is 80–85 °C. But the additional complexes obtained from compounds AlCl3, AlRCl2, AlR2Cl undergo hydrolysis. Hydroxychloride was obtained in the hydrolysis process of catalyst and then incurred the thermal processing. γ-Al2O3 is obtained from this mixture.
For the preparation of Mo, Ni/halloysite dispersed catalytic system by absorption method, halloysite is dried at 150 °C, and mixed with 5%(NH4)6Mo7·4H2O and 5% NiCl2 salt solution. The system is evaporated at 150 °C again and treated thermally in CVD (chemical vapor deposition) unit in argon medium during 4 h at + 850 °C.
-
2.
Ion-exchange method Halloysite is first dried for 2 h at 200 °C for preparing the catalyst by processing halloysite with HCl solvent. 100 g of 2.5 N HCl solution was added for releasing the metal mixtures settled in the dried halloysite pores. Accordingly the halloysite is mixed for 6 h by stirring with HCl solution of 1:10 ratio until a homogeneous solution is obtained. After completion of the process, the solution is washed with water until the chlorine ions are removed and then dried at 100 °C. H-shape halloysite is mixed at 1:10 molar ratio with water solution of metal chloride for 6 h at 90 °C, then it is dried at 100 °C. The dried sample was processed in argon medium for 4 h at 850 °C in CVD unit.
The catalyst of the process was dispersed by grinding up to a size of 25–50 micron. 1–2.5% of the catalyst was added to the fuel oil, homogenized at 80–90 °C, stirred until it became uniform to prepare the suspension. The hydrocracking process of the obtained uniform suspension was carried out at pressure of 0.5–6.0 MPa, temperature of 430 °C in the unit of “Heavy oil residues hydrocracking” (SPR-1) with modern software, that can be controlled online. The SPR-1 unit consists of a continuously automated CSTR (continuously stirred tank reactor) device. The CSTR system consists of a 100 mL internal-capacity reactor which is continuously filled with mazut, natural zeolite catalysts and hydrogen. Factors applied for the hydrocracking process carried out on the SPR-1 are these: maximum pressure and temperature for hydrocracking process are 60 bar and 525 °C, respectively.
The obtained product of hydrogenation process after filtration from catalytic additive was deposited on coke-like products as well as metals (Ni, V, Fe and Cu), and then was distilled with isolation of gasoline (start of boiling − 200 °C), diesel (200–360 °C) fractions and residue (> 360 °C).
Compositions of the products were analyzed using gas chromatography (AutoSystem XL, PerkinElmer). Chromatographic separations were achieved using helium gas in a Zebron ZB-1 capillary column coated with a dimethyl polysiloxane polymer as the stationary phase. Octane numbers were calculated on the basis of compositions of the gasoline fractions obtained from gas chromatograph (AutoSystem XL, PerkinElmer). Distillation fractions were determined by a crude oil distillation system (B/R Instruments Company, Easton, MD, USA) by ASTM D2892 and ASTM D86 standards. The sulfur content in gasoline and diesel fractions was determined by a SLFA-20 X-ray fluorescence sulfur in oil analyzer (Horiba Scientific) by the ASTM D4294 method. Densities of the gasoline and diesel fractions were measured with density meters (DMA 4500 M, Anton Paar) according to the ASTM D5002 method. The iodine number was determined by reacting the sample with iodine in chloroform solution and titrating residual iodine with sodium thiosulfate.
Results and discussion
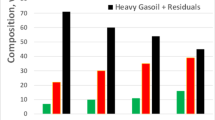
The analysis of the obtained results shows that the yield of the light color petroleum products changes depending on the catalyst. Thus, without catalyst, 46.6% (wt.) light petroleum products are obtained from the hydrocracking of fuel oil. The yield of the light petroleum products constitutes 53.0% which was increased by 7% by adding 2.5% halloysite catalyst to the system and, in this case, the yields of the gas, coke and residual fraction decrease properly from 13 to 10, 6.3 to 5.5 and 34.1 to 31.96%, while the yield of the gasoline increases from 16.2 up to 25.74%. When the process is conducted with halloysite catalyst modified with Mo and Ni by absorption method, the yield of the light petroleum products constitutes 63% (wt.) increasing by 10% in comparison with unmodified halloysite, but when using halloysite modified with Mo and Ni by ion-exchange method, it constitutes 83.0% increasing by 30%, and, hereby, the yields of the gas, coke and residual fraction decrease properly from 13 to 10%, 6.3 to 5.5%, 34.1 to 31.96%, while the yield of the gasoline increases from 16.2 up to 25.4%. As seen from the results, the yield of the light petroleum products is by 20% (wt.) more when using halloysite modified with Mo and Ni by ion-exchange method in comparison with halloysite modified by absorption method. This is related to almost complete removal of the aluminum oxide from the surface of the crystal particles when the halloysite is modified by ion-exchange method, where the natural halloysite mineral with dominating Al and Si oxides on surface layer is processed with HCl solution.
Molybdenum and nickel oxides substitute the aluminum oxide on the surface of the halloysite modified by the ion-exchange method. The surface layer of the halloysite crystals modified by ion-exchange method with Mo and Ni consists of 22.92% MoO, 18.03% NiO, and 48.76% SiO2. But in halloysite samples modified with Mo and Ni by absorption method, the aluminum oxide is preserved and metals are adsorbed on the surface of the halloysite. But the surface layer of the halloysite crystals modified by absorption method with Mo and Ni consists of 15.71% of Al2O3, 38.58% MoO2, 22.12% NiO, 14.32% SiO2. As seen in the sample modified by ion-exchange method, in the surface layers of the mineral, Mo and Ni elements were distributed more than in the sample modified by absorption method. Therefore, the maximum yield of the light petroleum products is obtained from hydrocracking process of fuel oil with halloysite modified by ion-exchange method equaling 83.0% (wt.) (Table 2). For comparison, it should be noted that, in the previous years, the authors of this article conducted the hydrocracking process of mazut in the presence of local natural aluminosilicate catalyst (Az 4), modified (via impregnation method) by transition metals. During the process, 63% (wt.) of light oil products were obtained [22].
As seen from Table 3, in hydrocarbon composition of gasoline fraction obtained from the hydrocracking of fuel oil without the catalyst, the amount of n-paraffins constitutes 42.98, iso-paraffins 29.71, and unsaturated hydrocarbons 12%. The amount of n-paraffins in the composition of gasoline obtained from hydrocracking process decreased from 42.98 to 26.37%, while the amount of iso-paraffins increased from 29.71 up to 32.76%, and unsaturated hydrocarbons decreased from 12 to 10% when 2.5% of the halloysite catalyst is added to the system. The amount of iso-paraffins properly increased up from 32.76 to 37.88 and 45.08%, while the amount of unsaturated hydrocarbons decreases from 10 to 6.69% and 4.33%, when the hydrocracking process was conducted with halloysite modified by absorption and ion-exchange methods. The amount of sulfur decreased from 0.0797 to 0.0512 and 0.0485% in gasoline fraction and in diesel fraction from 0.25 to 0.23 and 0.18%. The increase in the iso-structured saturated hydrocarbons amount, the reduction in unsaturated and aromatic hydrocarbons amount in gasoline composition can be explained by the modification of halloysite catalyst with transition metals (Ni, Mo). In other words, it is supposed that the hydrogenation and isomerization reactions occur more intensively with the impact of the transition metals. But the increase of the iso-structured saturated hydrocarbons amount, when comparing the sample modified by ion-exchange method with the sample modified by absorption method, can be explained by more distribution of Mo and Ni elements on the surface layers of halloysite.
NMR analysis
Structural characteristics of the gasoline fractions obtained from fuel oil hydrocracking in the presence of halloysite catalyst modified by absorption and ion-exchange methods with Mo, Ni and halloysite have been determined by NMR method (Table 4, Figs. 2, 3, 4, 5).
Structural characteristics of the diesel fractions obtained from fuel oil hydrocracking in the presence of halloysite catalyst modified by absorption and ion-exchange methods with Mo, Ni and Halloysite have been determined by NMR method (Table 5, Figs. 6, 7, 8, 9).
Results on composition of the gas extracted during the hydrocracking processes are shown in Table 6.
Conclusions
Thus, the following results were obtained from the hydrocracking process of fuel oil (in the presence of suspended halloysite modified by transition metals, through the two methods—absorption and ion-exchange methods):
When the hydrocracking of fuel oil is conducted without catalyst, 46.6% (wt.) of light petroleum products (gasoline and diesel fractions) are obtained.
When the process is carried out with halloysite modified by absorption method the yield of these products is 53%, in the case of using halloysite modified by ion-exchange method the yield is 83.0% (wt.).
As was noted in article, the increased yield during the process conducted in the presence of halloysite modified by ion-exchange method, can be explained so: when the halloysite (Al and Si oxides are dominant on the surface) modified by ion-exchange method is treated with HCl solution, aluminum oxide is almost completely being removed from the surface of the crystal particles. Aluminum oxide is replaced by Mo and Ni oxides on the surface of the halloysite modified by ion-exchange method. But, in halloysite samples modified by absorption method, Al oxide remains, and metals are absorbed on the surface of halloysite. In the sample modified by ion-exchange method, Mo and Ni elements are more distributed on the surface layers of halloysite in comparison with the sample modified by absorption method. That is why the maximum yield of the light petroleum products obtained from hydrocracking process of fuel oil with halloysite modified by ion-exchange method is 83.0% (wt.).
The gasoline fraction obtained from hydrocracking is characterized by stable low content of aromatic and unsaturated hydrocarbons. Its octane number is 75 p. by research method. Diesel fraction is also characterized by the low content of aromatic hydrocarbons that defines its high cetane number of 53–55 points. The analysis of the quality of gasoline and diesel fractions shows that, after the additional light hydrotreatment, the obtained products can be recommended as components to fuels and the gasoline fraction can be used as a component after hydrotreating or as a raw material for pyrolysis process.
References
Khadzhiyev S, Kadiyev Kh (2009) The future of petroleum deep refining. Chem J 9:34–37
Shmelkova O, Gulyaeva L, Khavkin V et al (2013) Development of destructive processes for oil residues treatment in Russia and abroad. World Pet Prod 9:15–19
Popkova V, Chernysheva E (2016) Development of configurations for hydroprocessing of heavy oil feedstocks. In: IX International industrial and economic form “Combining strategy: solving the urgent tasks of the oil and gas and petrochemical complexes at the present stage”. Forum materials, pp 76–77
Ya Kon, Zelkin E, Shershun V (1986) Oil refining and petrochemical industry abroad. Khimiya, Moscow
Antonovov M (2009) Refining of petroleum residues Refining of petroleum residues at the plants of the group of “Lukoyl”. Experience and perspectives. World Oil Prod 14:6–9
Berg G, Khabibullin S (1986) Catalytic hydrorefining of oil residues. Khimiya, Leningrad
Zorina G (1988) Abstracts of reports on refining at the XII World Oil Congress. Chem Technol Fuels Oils 1:42–47
Ishihara A, Itoh T, Nasu H et al (2013) Hydrocracking of 1-methylnaphthalene decahydronaphthalene mixture catalyzed by zeolite-alumina composite supported NiMo catalysts. Fuel Process Technol 116:222–227
Wang D, Xu L, Wu P (2014) Hierarchical, core–shell meso-ZSM-5@mesoporous aluminosilicate-supported Pt nanoparticles for bifunctional hydrocracking. J Mater Chem A 2:15535–15545
Pereyma VYu, Klimov OV, Budukva SV et al (2015) Hydrocracking of vacuum gas oil in the presence of catalysts NiMo/Al2O3-amorphous aluminosilicates and NiW/Al2O3-amorphous aluminosilicates. J Appl Chem 88:1722–1728
Okunev AG, Parkhomchukh VV, Lysikov AI (2015) Catalytic hydroprocessing of heavy crude oil. Advances in chemistry. Rev J Chem 84:981–999
Alvarez F, Ribeiro FR, Perot G et al (1996) Hydroisomerization and hydrocracking of alkanes. J Catal 2:179–189
Bates T, Hildebrand F, Swineford A (1950) Morphology and structure of endellite and halloysite. Am Miner 35:463–484
Joussein E, Petit S, Churchman J (2005) Halloysite clay minerals—a review. Clay Miner 40:383–390
Price R, Gaber B, Lvov Y (2001) In-vitro release characteristics of tetracycline HCl, khellin and nicotinamide adenine dinucleotide from halloysite; a cylindrical mineral. J Microencapsul 18:713–723
Guo B, Zou Q, Lei Y et al (2008) Application of inhibitor loaded halloysite nanotubes in active anticorrosive coatings. New Chem Mater 36:32–40
Liu M, Guo B, Du M (2007) Properties of halloysite nanotube-epoxy resin hybrids and the interfacial reactions in the systems. Nanotechnology 18:1–9
Robson H (1978) Synthetic halloysites as hydrocarbon conversion catalysts. 4098676. U.S. Patent
Santilli, D (1982) Residual oil processing catalysts. 4358400. U.S. Patent
Gary J, Handwerk G (2001) Petroleum refining technology and economics, vol 11, 4th edn. Marcel Dekker Inc., New York, p 106
Offutt W, Whitaker A (1956) Catalytic conversion process employing as catalyst, a halloysite clay activated with magnesium oxide. 27440 U.S. Patent 56
Abbasov VM, Ibrahimov HC, Mukhtarova GS et al (2017) Influence of temperature to the hydrocracking of mazut in the presence of a suspended aluminosilicates catalyst. PPOR 18:13–17
Acknowledgements
The presented research work was done corresponding to scientific research plans (State registration number 0113Az2039).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hasanova, A., Alizade, A., Ahmadova, R. et al. Hydrocracking process of fuel oil using halloysite modified by different methods. Appl Petrochem Res 9, 199–209 (2019). https://doi.org/10.1007/s13203-019-00234-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-019-00234-7