Abstract
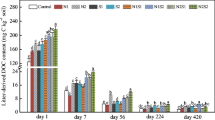
Litter types and soil properties can affect litter decomposition rate and litter carbon (C) transformation, consequently regulating soil C cycling. Yet, litter C transformations of leaves and roots of invasive plants are poorly understood, which limits our understanding of the role of plant residues in soil C sequestration during plant invasion. In a laboratory incubation experiment lasting for 153 days, we used two types of soil which were collected from invasive S. alterniflora and native Phragmites australis marshlands, and traced the transformation of 13C from leaf and root litter of invasive Spartina alterniflora into CO2, soil-dissolved organic C (DOC), microbial biomass C (MBC), and soil organic C (SOC). The leaf litter of S. alterniflora decomposed faster than root litter, resulting in higher soil respiration and higher transformation of litter-derived 13C into CO2, MBC, and DOC. Although the root litter of S. alterniflora decomposed slowly, SOC comprised up to 24% of litter-derived 13C. Furthermore, the litter C transformations of S. alterniflora showed a positive home-field advantage effect. Soil respiration, MBC, fractions of litter-derived 13C in Gram-negative bacteria, 13C recovered in CO2, DOC, and SOC were higher in the soil colonized by S. alterniflora than in the soil colonized by P. australis, with the home-field advantage effect being more pronounced in root than leaf litter treatments. Therefore, litter type and soil source had differential impacts on litter C transformation patterns of S. alterniflora and the root litter of invasive S. alterniflora played an important role in SOC formation and C sequestration in soils from its invaded ecosystems.


Similar content being viewed by others
References
Ayres E, Dromph KM, Bardgett RD (2006) Do plant species encourage soil biota that specialise in the rapid decomposition of their litter? Soil Biol Biochem 38:183–186
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH (2009) Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol Biochem 41:606–610
Balesdent J, Mariotti A (1996) Measurement of soil organic matter turnover using 13C natural abundance. In: Boutton TW, Yamasaki S (eds) Mass spectrometry of soils. Marcel Dekker, New York, pp 83–111
Benner R, Fogel ML, Sprauge EK, Hodson RE (1987) Depletion of 13C in lignin and its implications for stable carbon isotope studies. Nature 329:708–710
Berg B, Staaf H (1981) Leaching, accumulation and release of nitrogen in decomposing forest litter. Ecol Bull (Stockholm) 33:163–178
Bird JA, Torn MS (2006) Fine roots vs. needles: a comparison of 13C and 15N dynamics in a ponderosa pine forest soil. Biogeochemistry 79:361–382
Bird JA, Kleber M, Torn MS (2008) 13C and 15N stabilization dynamics in soil organic matter fractions during needle and fine root decomposition. Org Geochem 39:465–477
Bloomfield J, Vogt KA, Vogt DJ (1993) Decay rate and substrate quality of fine roots and foliage of two tropical tree species in the Luquillo experimental Forest, Puerto Rico. Plant Soil 150:233–245
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: phospholipid fatty acid profiles and substrate utilization patterns. Microb Ecol 35:265–278
Bradford MA, Strickland MS, DeVore JL, Maerz JC (2012) Root carbon flow from an invasive plant to belowground foodwebs. Plant Soil 359:233–244
Brüggemann N, Gessler A, Kayler Z, Keel SG, Badeck F, Barthel M, Boeckx P, Buchmann N, Brugnoli E, Esperschütz J, Gavrichkova O, Ghashghaie J, Gomez-Casanovas N, Keitel C, Knohl A, Kuptz D, Palacio S, Salmon Y, Uchida Y, Bahn M (2011) Carbon allocation and carbon isotope fluxes in the plant-soil-atmosphere continuum: a review. Biogeosciences 8:3457–3489
Connin SL, Feng X, Virginia RA (2001) Isotopic discrimination during long-term decomposition in an arid land ecosystem. Soil Biol Biochem 33:41–51
Cotrufo MF, del Galdo I, Piermatteo D (2009) Litter decomposition: concepts, methods and future perspectives. In: Kutsch WL, Bahn M, Heinemeyer A (eds) Soil carbon dynamics: an integrated methodology. Cambridge University Press, Cambridge, pp 76–90
Crow SE, Lajtha K, Bowden RD, Yano Y, Brant JB, Caldwell BA, Sulzman EW (2009) Increased coniferous needle inputs accelerate decomposition of soil carbon in an old-growth forest. Forest Ecol Man 258:2224–2232
Delgado-Baquerizo M, García-Palacios P, Milla R, Gallardo A, Maestre FT (2015) Soil characteristics determine soil carbon and nitrogen availability during leaf litter decomposition regardless of litter quality. Soil Biol Biochem 81:134–142
Esperschütz J, Welzl G, Schreiner K, Buegger F, Munch JC, Michael S (2011) Incorporation of carbon from decomposing litter of two pioneer plant species into microbial communities of the detritusphere. FEMS Microbiol Lett 320:48–55
Fernandez I, Mahieu N, Cadisch G (2003) Carbon isotopic fractionation during decomposition of plant materials of different quality. Global Biogeochem Cy 17:1075
Findlay SEG, Dye S, Kuehn KA (2002) Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22:616–625
Freschet GT, Aerts R, Cornelissen JHC (2012) Multiple mechanisms for trait effects on litter decomposition: moving beyond home-field advantage with a new hypothesis. J Ecol 100:619–630
Freschet GT, Cornwell WK, Wardle DA, Elumeeva TG, Liu W, Jackson BG, Onipchenko VG, Soudzilovskaia NA, Tao J, Cornelissen JHC (2013) Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide. J Ecol 101:943–952
Frostegård A, Bååth E (1996) The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol Fert Soils 22:59–65
Gedan KB, Silliman BR, Bertness MD (2009) Centuries of human-driven change in salt marsh ecosystems. Annu Rev Mar Sci 1:117–141
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Chang Biol 6:751–765
Hansson K, Kleja DB, Kalbitz K, Larsson H (2010) Amounts of carbon mineralised and leached as DOC during decomposition of Norway spruce needles and fine roots. Soil Biol Biochem 42:178–185
Heitkamp F, Jacobs A, Jungkunst HF, Heinze S, Wendland M, Kuzyakov Y (2012) Processes of soil carbon dynamics and ecosystem carbon cycling in a changing world. In: Lal R, Lorenz K, Hüttl RF, Schneider BU, von Braun J (eds) Recarbonization of the biosphere ecosystems and the global carbon cycle. Springer, Dordrecht, pp 395–428
Hooker TD, Stark JM (2008) Soil C and N cycling in three semiarid vegetation types: response to an in situ pulse of plant detritus. Soil Biol Biochem 40:2678–2685
Huangfu C, Hui D, Qi X, Li K (2019) Plant interactions modulate root litter decomposition and negative plant-soil feedback with an invasive plants. Plant Soil 437:179–194
Hunt HW, Ingham ER, Coleman DC, Elliott ET, Reid CPP (1988) Nitrogen limitation of production and decomposition in prairie, mountain meadow, and pine forest. Ecology 69:1009–1016
Incerti G, Cartenì F, Cesarano G, Sarker TC, Abd EI-Gawad AM, D’Ascoli RD, Bonanomi G, Giannino F (2018) Faster N release, but not C loss, from leaf litter of invasives compared to native species in Mediterranean ecosystems. Front Plant Sci 9:354
Jackson RB, Lajtha K, Crow SE, Hugelius G, Kramer MG, Piñeiro G (2017) The ecology of soil carbon: pools, vulnerabilities, and biotic and abiotic controls. Annu Rev Ecol Evol Syst 48:419–445
Kammer A, Hagedorn F (2011) Mineralisation, leaching and stabilisation of 13C-labelled leaf and twig litter in a beech forest soil. Biogeosciences 8:2195–2208
Kramer C, Gleixner G (2006) Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol Biochem 38:3267–3278
Kramer C, Trumbore S, Fröberg M, Cisneros Dozal LM, Zhang D, Xu X, Santos GM, Hanson PJ (2010) Recent (< 4 year old) leaf litter is not a major source of microbial carbon in a temperate forest mineral soil. Soil Biol Biochem 42:1028–1037
Kramer S, Dibbern D, Moll J, Huenninghaus M, Koller R, Krueger D, Marhan S, Urich T, Wubet T, Bonkowski M, Buscot F, Lueders T, Kandeler E (2016) Resource partitioning between bacteria, fungi, and protists in the detritusphere of an agricultural soil. Front Microbiol 7:1524
Kuzyakov Y, Friedel JL, Stahr K (2000) Review of mechanisms and quantification of priming effects. Soil Biol Biochem 32:1485–1498
Kuzyakov Y, Siniakina SV, Ruehlmann J, Domanski G, Stahr K (2002) Effect of nitrogen fertilisation on below-ground carbon allocation in lettuce. J Sci Food Agr 82:1432–1441
Li B, Liao C, Zhang X, Chen H, Wang Q, Chen Z, Gan X, Wu J, Zhao B, Ma Z, Cheng X, Jiang L, Chen J (2009) Spartina alterniflora invasions in the Yangtze River estuary, China: an overview of current status and ecosystem effects. Ecol Eng 35:511–520
Liao CZ, Luo YQ, Fang CM, Chen JK, Li B (2008) Litter pool sizes, decomposition, and nitrogen dynamics in Spartina alterniflora-invaded and native coastal marshlands of the Yangtze estuary. Oecologia 156:589–600
Lummer D, Scheu S, Butenschoen O (2012) Connecting litter quality, microbial community and nitrogen transfer mechanisms in decomposing litter mixtures. Oikos 121:1649–1655
Marchante E, Kjoller A, Struwe S, Freitas H (2009) Soil recovery after removel of the N2-fixing invasive Acacia longifolia: consequences for ecosystem restoration. Biol Invasions 11:813–823
Marhan S, Kandeler E, Rein S, Fangmeier A, Niklaus PA (2010) Indirect effects of soil moisture reverse soil C sequestration responses of a spring wheat agroecosystem to elevated CO2. Glob Chang Biol 16:469–483
McLeod E, Chmura GL, Bouillon S, Salm R, Björk M, Duarte CM, Lovelock CE, Schlesinger WH, Silliman BR (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front Ecol Environ 9:552–560
Morgan JAW, Winstanley C (1997) Microbial biomarkers. In: van Elsas JD, Trevors JT, Wellington EMH (eds) Modern soil microbiology. Marcel Dekker, New York, pp 331–352
Müller K, Marhan S, Kandeler E, Poll C (2017) Carbon flow from litter through soil microorganisms: from incorporation rates to mean residence times in bacteria and fungi. Soil Biol Biochem 115:187–196
Osono T, Takeda H, Azuma J (2007) Carbon isotope dynamics during leaf litter decomposition with reference to lignin fractions. Ecol Res 23:51–55
Ostertag R, Hobbie SE (1999) Early stages of root and leaf decomposition in Hawaiian forests: effects of nutrient availability. Oecologia 121:564–573
Porazinska DL, Bardgett RD, Blaauw MB, Hunt HW, Parsons AN, Seastedt TR, Wall DH (2003) Relationships at the aboveground-belowground interface: plants, soil biota, and soil processes. Ecol Monogr 73:377–395
Preusser S, Marhan S, Poll C, Kandeler E (2017) Microbial community response to changes in substrate availability and habitat conditions in a reciprocal subsoil transfer experiment. Soil Biol Biochem 105:138–152
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil 269:341–356
Rubino M, Dungait JAJ, Evershed RP, Bertolini T, De Angelis P, D’Onofrio A, Lagomarsino A, Lubritto C, Merola A, Terrasi F (2010) Carbon input belowground is the major C flux contributing to leaf litter mass loss: evidences from a 13C labelled-leaf litter experiment. Soil Biol Biochem 42:1009–1016
Sayer EJ, Heard MS, Grant HK, Marthews TR, Tanner EVJ (2011) Soil carbon release enhanced by increased tropical forest litterfall. Nat Clim Chang 1:304–307
Schweizer M, Fear J, Cadisch G (1999) Isotopic (13C) fractionation during plant residue decomposition and its implications for soil organic matter studies. Rapid Commun Mass Sp 13:1284–1290
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Standish RJ, Williams PA, Robertson AW, Scott NA, Hedderley DI (2004) Invasion by a perennial herb increases decomposition rate and alters nutrient availability in warm temperate lowland forest remnants. Biol Invasions 6:71–81
Steffens C, Helfrich M, Joergensen RG, Eissfeller V, Flessa H (2015) Translocation of 13C-labeled leaf or root litter carbon of beech (Fagus sylvatica L.) and ash (Fraxinus excelsior L.) during decomposition - a laboratory incubation experiment. Soil Biol Biochem 83:125–137
Strickland MS, Lauber C, Fierer N, Bradford MA (2009a) Testing the functional significance of microbial community composition. Ecology 90:441–451
Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA (2009b) Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculum characteristics. Funct Ecol 23:627–636
Sun T, Hobbie SE, Berg B, Zhang H, Wang Q, Wang Z, Hattenschwiler S (2018) Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. P Nati Acad Sci USA 115:10392–10397
Trumbore SE, Czimczik CI (2008) An uncertain future for soil carbon. Science 321:1455–1456
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vivanco L, Austin AT (2006) Intrinsic effects of species on leaf litter and root decomposition: a comparison of temperate grasses from North and South America. Oecologia 150:97–107
Wallenstein MD, Hess AM, Lewis MR, Steltzer H, Ayres E (2010) Decomposition of aspen leaf litter results in unique metabolomes when decomposed under different tree species. Soil Biol Biochem 42:484–490
Windham L, Ehrenfeld JG (2003) Net impact of a plant invasion on nitrogen-cycling processes within a brackish tidal marsh. Ecol Appl 13:883–897
Xia M, Talhelm AF, Pregitzer KS (2015) Fine roots are the dominant source of recalcitrant plant litter in sugar maple-dominated northern hardwood forests. New Phytol 208:715–726
Yan J, Wang L, Hu Y, Tsang YF, Zhang Y, Wu J, Fu X, Sun Y (2018) Plant litter composition selects different soil microbial structures and in turn drives different litter decomposition pattern and soil carbon sequestration capability. Geoderma 319:194–203
Zelles L, Palojärvi A, Kandeler E, Lützow MV, Winter K, Bai QY (1997) Changes in soil microbial properties and phospholipid fatty acid fractions after chloroform fumigation. Soil Biol Biochem 29:1325–1336
Zhang Y, Ding W, Luo J, Donnison A (2010) Changes in soil organic carbon dynamics in an eastern Chinese coastal wetland following invasion by a C4 plant Spartina alterniflora. Soil Biol Biochem 42:1712–1720
Zhang P, Nie M, Li B, Wu J (2017) The transfer and allocation of newly fixed C by invasive Spartina alterniflora and native Phragmites australis to soil microbiota. Soil Biol Biochem 113:231–239
Zhang P, Li B, Wu J, Hu S (2019) Invasive plants differentially affect soil biota through litter and rhizosphere pathways: a meta-analysis. Ecol Lett 22:200–210
Zhao H, Huang G, Ma J, Li Y, Tang L (2013) Decomposition of aboveground and root litter for three desert herbs: mass loss and dynamics of mineral nutrients. Biol Fert Soils 50:745–753
Acknowledgments
We acknowledge Prof. Ming Nie for his advice to the experimental design, Jun Yan and Jinquan Li for their help in litter preparation and measurements of microbial biomass carbon. We are grateful to Dr. Sarah Zieger and Dr. Xianshan Zhu for their help on isotope analyses.
Funding
This study was financially supported by the National Key R&D Program of China (2017YFC1200103), National Natural Science Foundation of China (Grant No. 41371258, 31570513), Science and Technology Department of Shanghai (18DZ1206507), and the Fundamental Research Funds for the Central Universities (SCU2019D013).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. PZ performed the material preparation, data collection, and analysis and wrote the first draft of manuscript, and all authors contributed substantially to revisions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 19 kb).
Rights and permissions
About this article
Cite this article
Zhang, P., Scheu, S., Li, B. et al. Litter C transformations of invasive Spartina alterniflora affected by litter type and soil source. Biol Fertil Soils 56, 369–379 (2020). https://doi.org/10.1007/s00374-019-01429-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-019-01429-9