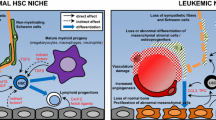
Abstract
Hematopoietic stem cells (HSCs) exist in a close contact with their specific microenvironment, called a niche, which supports the HSC function and significantly influences the HSC properties. The existence of the HSC niche, which was proposed as a purely theoretical concept in 1978, finds increasing experimental evidence and is now generally accepted by specialists in the field of hematopoiesis. The review briefly describes various cell components of the HSC niche in bone marrow, considers the metabolic states of the niche and HSCs, and discusses other aspects of niche biology. Increasing knowledge of the HSC niche will help to create in vitro cell models of the HSC niche, to modulate the HSC properties, and to achieve multifold HSC expansion in culture for further applications in therapeutic practice.

Similar content being viewed by others
REFERENCES
Maximov A.A. 1909. Der Lymphozyt als gemeinsame Stammzelle der verschiedenen Blutelemente in der embryonalen Entwicklung und im postfetalen Leben der Säugetiere. Folia Haematol.8, 125–134.
Becker A.J., McCulloch E.A., Till J.E. 1963. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 197, 452–454.
Schofield R. 1978. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 4 (1–2), 7–25.
Zhang J., Niu C., Ye L., Huang H., He X., Tong W.G., Ross J., Haug J., Johnson T., Feng J.Q., Harris S., Wiedemann L.M., Mishina Y., Li L. 2003. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 425 (6960), 836–841.
Calvi L.M., Adams G.B., Weibrecht K.W., Weber J.M., Olson D.P., Knight M.C., Martin R.P., Schipani E., Divieti P., Bringhurst F.R., Milner L.A., Kronenberg H.M., Scadden D.T. 2003. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 425 (6960), 841–846.
Visnjic D., Kalajzic Z., Rowe D.W., Katavic V., Lorenzo J., Aguila H.L. 2004. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 103 (9), 3258–3264.
Zhu J., Garrett R., Jung Y., Zhang Y., Kim N., Wang J., Joe G.J., Hexner E., Choi Y., Taichman R.S., Emerson S.G. 2007. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 109 (9), 3706–3712.
Kiel M.J., Yilmaz O.H., Iwashita T., Yilmaz O.H., Terhorst C., Morrison S.J. 2005. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 121 (7), 1109–1121.
Friedenstein A.J., Deriglasova U.F., Kulagina N.N., Panasuk A.F., Rudakowa S.F., Luriá E.A., Ruadkow I.A. 1974. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 2 (2), 83–92.
Chan C.K., Chen C.C., Luppen C.A., Kim J.B., DeBoer A.T., Wei K., Helms J.A., Kuo C.J., Kraft D.L., Weissman I.L. 2009. Endochondral ossification is required for hematopoietic stem-cell niche formation. Nature. 457 (7228), 490–494.
Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P.G., Riminucci M., Bianco P. 2007. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 131 (2), 324–336.
Méndez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma’ayan A., Enikolopov G.N., Frenette P.S. 2010. Mesenchymal and hematopoietic stem cells form a unique bone marrow niche. Nature. 466 (7308), 829–834.
Katayama Y., Battista M., Kao W.M., Hidalgo A., Peired A.J., Thomas S.A., Frenette P.S. 2006. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell.124 (2), 407–421.
Omatsu Y., Sugiyama T., Kohara H., Kondoh G., Fujii N., Kohno K., Nagasawa T. 2010. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 33 (3), 387–399.
Ding L., Saunders T.L., Enikolopov G., Morrison S.J. 2012. Endothelial and perivascular cells maintain hematopoietic stem cells. Nature. 481 (7382), 457–462.
Zhou B.O., Yue R., Murphy M.M., Peyer J.G., Morrison S.J. 2014. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 15 (2), 154–168.
Kunisaki Y., Bruns I., Scheiermann C., Ahmed J., Pinho S., Zhang D., Mizoguchi T., Wei Q., Lucas D., Ito K., Mar J.C., Bergman A., Frenette P.S. 2013. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 502 (7473), 637–643.
Asada N., Kunisaki Y., Pierce H., Wang Z., Fernandez N.F., Birbrair A., Ma’ayan A., Frenette P.S. 2017. Differential cytokine contributions of perivascular hematopoietic stem cell niches. Nat. Cell. Biol. 19 (3), 214–223.
Hooper A.T., Butler J.M., Nolan D.J., Kranz A., Iida K., Kobayashi M., Kopp H.G., Shido K., Petit I., Yanger K., James D., Witte L., Zhu Z., Wu Y., Pytowski B., et al. 2009. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 4 (3), 263–274.
Himburg H.A., Harris J.R., Ito T., Daher P., Russell J.L., Quarmyne M., Doan P.L., Helms K., Nakamura M., Fixsen E., Herradon G., Reya T., Chao N.J., Harroch S., Chute J.P. 2012. Pleiotrophin regulates the retention and self-renewal of hematopoietic stem cells in the bone marrow vascular niche. Cell Rep. 2 (4), 964–975.
Himburg H.A., Termini C.M., Schlussel L., Kan J., Li M., Zhao L., Fang T., Sasine J.P., Chang V.Y., Chute J.P. 2018. Distinct bone marrow sources of pleiotrophin control hematopoietic stem cell maintenance and regeneration. Cell Stem Cell.23 (3), 370–381.
Chow A., Lucas D., Hidalgo A., Méndez-Ferrer S., Hashimoto D., Scheiermann C., Battista M., Leboeuf M., Prophete C., van Rooijen N., Tanaka M., Merad M., Frenette P.S. 2011. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med.208 (2), 261–271.
Albiero M., Poncina N., Ciciliot S., Cappellari R., Menegazzo L., Ferraro F., Bolego C., Cignarella A., Avogaro A., Fadini G.P. 2015. Bone marrow macrophages contribute to diabetic stem cell mobilopathy by producing oncostatin M. Diabetes.64 (8), 2957–2968.
Ludin A., Itkin T., Gur-Cohen S., Mildner A., Shezen E., Golan K., Kollet O., Kalinkovich A., Porat Z., D’Uva G., Schajnovitz A., Voronov E., Brenner D.A., Apte R.N., Jung S., Lapidot T. 2012. Monocytes-macrophages that express α-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat. Immunol. 13 (11), 1072–1082.
Winkler I.G., Sims N.A., Pettit A.R., Barbier V., Now-lan B., Helwani F., Poulton I.J., van Rooijen N., Alexander K.A., Raggatt L.J., Lévesque J.P. 2010. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 116 (23), 4815–4828.
Kollet O., Dar A., Shivtiel S., Kalinkovich A., Lapid K., Sztainberg Y., Tesio M., Samstein R.M., Goichberg P., Spiegel A., Elson A., Lapidot T. 2006. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 12 (6), 657–664.
Bruns I., Lucas D., Pinho S., Ahmed J., Lambert M.P., Kunisaki Y., Scheiermann C., Schiff L., Poncz M., Bergman A., Frenette P.S. 2014. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat. Med. 20 (11), 1315–1320.
Zhao M., Perry J.M., Marshall H., Venkatraman A., Qian P., He X.C., Ahamed J., Li L. 2014. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat. Med. 20 (11), 1321–1326.
Nakamura-Ishizu A., Takubo K., Fujioka M., Suda T. 2014. Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem. Biophys. Res. Commun.454 (2), 353–357.
Fujisaki J., Wu J., Carlson A.L., Silberstein L., Putheti P., Larocca R., Gao W., Saito T.I., Lo Celso C., Tsuyuzaki H., Sato T., Côté D., Sykes M., Strom T.B., Scadden D.T., Lin C.P. 2011. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 474 (7350), 216–219.
Hirata Y., Furuhashi K., Ishii H., Li H.W., Pinho S., Ding L., Robson S.C., Frenette P.S., Fujisaki J. 2018. CD150high bone marrow Tregs maintain hematopoietic stem cell quiescence and immune privilege via adenosine. Cell Stem Cell. 22 (3), 445–453.
Schürch C.M., Riether C., Ochsenbein A.F. 2014. Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell. 14 (4), 460–472.
Suda T., Takubo K., Semenza G.L. 2011. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 9 (4), 298–310.
Parmar K., Mauch P., Vergilio J.A., Sackstein R., Down J.D. 2007. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc. Natl. Acad. Sci. U. S. A.104 (13), 5431–5436.
Takubo K., Goda N., Yamada W., Iriuchishima H., Ikeda E., Kubota Y., Shima H., Johnson R.S., Hirao A., Suematsu M., Suda T. 2010. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell. 7 (3), 391–402.
Vukovic M., Sepulveda C., Subramani C., Guitart A.V., Mohr J., Allen L., Panagopoulou T.I., Paris J., Lawson H., Villacreces A., Armesilla-Diaz A., Gezer D., Holyoake T.L., Ratcliffe P.J., Kranc K.R. 2016. Adult hematopoietic stem cells lacking Hif-1α self-renew normally. Blood. 127 (23), 2841–2846.
Takubo K., Nagamatsu G., Kobayashi C.I., Nakamura-Ishizu A., Kobayashi H., Ikeda E., Goda N., Rahimi Y., Johnson R.S., Soga T., Hirao A., Suematsu M., Suda T. 2013. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 12 (1), 49–61.
Spencer J.A., Ferraro F., Roussakis E., Klein A., Wu J., Runnels J.M., Zaher W., Mortensen L.J., Alt C., Turcotte R., Yusuf R., Côté D., Vinogradov S.A., Scadden D.T., Lin C.P. 2014. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 508 (7495), 269–273.
Zuo L., Zhou T., Pannell B.K., Ziegler A.C., Best T.M. 2015. Biological and physiological role of reactive oxygen species—the good, the bad and the ugly. Acta Physiol. (Oxford). 214 (3), 329–348
Juntilla M.M., Patil V.D., Calamito M., Joshi R.P., Birnbaum M.J., Koretzky G.A. 2010. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood. 115 (20), 4030–4038.
Ito K., Hirao A., Arai F., Matsuoka S., Takubo K., Hamaguchi I., Nomiyama K., Hosokawa K., Sakurada K., Nakagata N., Ikeda Y., Mak T.W., Suda T. 2004. Regulation of oxidative stress by ATM is required for self-renewal of hematopoietic stem cells. Nature. 431 (7011), 997–1002.
Miyamoto K., Araki K.Y., Naka K., Arai F., Takubo K., Yamazaki S., Matsuoka S., Miyamoto T., Ito K., Ohmura M., Chen C., Hosokawa K., Nakauchi H., Nakayama K., Nakayama K.I., et al. 2007. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 1 (1), 101–112.
Warr M.R., Binnewies M., Flach J., Reynaud D., Garg T., Malhotra R., Debnath J., Passegué E. 2013. FOXO3A directs a protective autophagy program in hematopoietic stem cells. Nature. 494 (7437), 323–327.
Ludin A., Gur-Cohen S., Golan K., Kaufmann K.B., Itkin T., Medaglia C., Lu X.J., Ledergor G., Kollet O., Lapidot T. 2014. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid. Redox Signal. 21 (11), 160–1619.
Tesio M., Golan K., Corso S., Giordano S., Schajnovitz A., Vagima Y., Shivtiel S., Kalinkovich A., Caione L., Gammaitoni L., Laurenti E., Buss E.C., Shezen E., Itkin T., Kollet O., et al. 2011. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood. 117 (2), 419–428.
Zalba G., Fortuño A., Orbe J., San José G., Moreno M.U., Belzunce M., Rodríguez J.A., Beloqui O., Páramo J.A., Díez J. 2007. Phagocytic NADPH oxidase-dependent superoxide production stimulates matrix metalloproteinase-9: Implications for human atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27 (3), 587–593.
Walkley C.R., Olsen G.H., Dworkin S., Fabb S.A., Swann J., McArthur G.A., Westmoreland S.V., Chambon P., Scadden D.T., Purton L.E. 2007. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell. 129 (6), 1097–1110.
Kim Y.W., Koo B.K., Jeong H.W., Yoon M.J., Song R., Shin J., Jeong D.C., Kim S.H., Kong Y.Y. 2008. Defective Notch activation in microenvironment leads to myeloproliferative disease. Blood. 112 (12), 4628–4638.
Sipkins D.A., Wei X., Wu J.W., Runnels J.M., Côté D., Means T.K., Luster A.D., Scadden D.T., Lin C.P. 2005. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 435 (7044), 969–973.
Pitt L.A., Tikhonova A.N., Hu H., Trimarchi T., King B., Gong Y., Sanchez-Martin M., Tsirigos A., Littman D.R., Ferrando A.A., Morrison S.J., Fooksman D.R., Aifantis I., Schwab S.R. 2015. CXCL12-producing vascular endothelial niches control acute T cell leukemia maintenance. Cancer Cell. 27 (6), 755–768.
Janel A., Dubois-Galopin F., Bourgne C., Berger J., Tarte K., Boiret-Dupré N., Boisgard S., Verrelle P., Déchelotte P., Tournilhac O., Berger M.G. 2014. The chronic lymphocytic leukemia clone disrupts the bone marrow microenvironment. Stem Cells Dev. 23 (24), 2972–2982.
Vanegas N.P., Vernot J.P. 2017. Loss of quiescence and self-renewal capacity of hematopoietic stem cell in an in vitro leukemic niche. Exp. Hematol. Oncol. 6, 2.
Pinho S., Marchand T., Yang E., Wei Q., Nerlov C., Frenette P.S. 2018. Lineage-biased hematopoietic stem cells are regulated by distinct niches. Dev. Cell.44 (5), 634–641.
Ding L., Morrison S.J. 2013. Hematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 495 (7440), 231–235.
Greenbaum A., Hsu Y.M., Day R.B., Schuettpelz L.G., Christopher M.J., Borgerding J.N., Nagasawa T., Link D.C. 2013. CXCL12 in early mesenchymal progenitors is required for hematopoietic stem-cell maintenance. Nature. 495 (7440), 227—230.
Hérault A., Binnewies M., Leong S., Calero-Nieto F.J., Zhang S.Y., Kang Y.A., Wang X., Pietras E.M., Chu S.H., Barry-Holson K., Armstrong S., Göttgens B., Passegué E. 2017. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature. 544 (7648), 53–58.
Shimoto M., Sugiyama T., Nagasawa T. 2017. Numerous niches for hematopoietic stem cells remain empty during homeostasis. Blood. 129 (15), 2124–2131.
Funding
This work was supported by the Russian Science Foundation (project no. 18-14-00300).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author declares that he has no conflict of interest. This article does not contain any studies involving animals or human subjects performed by the author.
Additional information
Translated by T. Tkacheva
Abbreviations: HSC, hematopoietic stem cell; BM, bone marrow.
Rights and permissions
About this article
Cite this article
Belyavsky, A.V. Niches of Hematopoietic Stem Cells in Bone Marrow. Mol Biol 53, 889–895 (2019). https://doi.org/10.1134/S0026893319060025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893319060025