Abstract
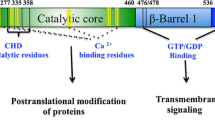
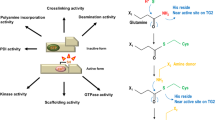
Transglutaminase 2 (TGase2) is involved in a variety of cellular processes and diseases via its transamidase and kinase activities, which are regulated by conformational changes induced by the binding of nucleotides and divalent cations. However, due to the lack of an appropriate assay system, the function of critical amino acid residues in the regulation of both activities is unclear. Thus, we designed site-directed TGase2 mutants that were then used in protein arrays to investigate the effects of the mutations on the regulation of TGase2 transamidase and kinase activities. We found that the Lys444Ala mutation, but not the Arg580Lys and Lys663Ala mutations, completely inhibited the transamidase activity. Additionally, the mutations at Lys444 and Lys663 inhibited the kinase activity by 27% and 48%, respectively, but the mutations at Cys277 and Arg580 had no effect. Furthermore, a kinetic analysis of the transamidation reaction revealed that the Lys663Ala mutation increased the affinity of TGase2 for the substrate fibrinogen. Thus, this array-based approach would be helpful for investigation of amino acids responsible for regulation of the TGase2 transamidase and kinase activities and the pathogenesis of TGase2- mediated diseases.






Similar content being viewed by others
References
Kwon, M.H., Jung, J.W., Jung, S.H., Park, J.Y., Kim, Y.M. & Ha, K.S. Quantitative and rapid analysis of transglutaminase activity using protein arrays in mammalian cells. Mol. Cells 27, 337–343 (2009).
Nurminskaya, M.V. & Belkin, A.M. Cellular functions of tissue transglutaminase. Int. Rev. Cell Mol. Biol. 294, 1–97 (2012).
Mishra, S. & Murphy, L.J. Phosphorylation of transglutaminase 2 by PKA at Ser216 creates 14-3-3 binding sites. Biochem. Biophys. Res. Commun. 347, 1166–1170 (2006).
Park, D., Choi, S.S. & Ha, K.S. Transglutaminase 2: a multi-functional protein in multiple subcellular compartments. Amino Acids 39, 619–631 (2010).
Fesus, L. & Piacentini, M. Transglutaminase 2: an enigmatic enzyme with diverse functions. Trends Biochem. Sci. 27, 534–539 (2002).
Collighan, R.J. & Griffin, M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids 36, 659–670 (2009).
Jung, S.H., Lee, K., Kong, D.H., Kim, W.J., Kim, Y.M. & Ha, K.S. Integrative proteomic profiling of protein activity and interactions using protein arrays. Mol. Cell. Proteomics 11, 1167–1176 (2012).
Bhatt, M.P., Lim, Y.C., Hwang, J., Na, S., Kim, Y. M. & Ha, K.S. C-Peptide Prevents Hyperglycemia- Induced Endothelial Apoptosis Through Inhibition of Reactive Oxygen Species-Mediated Transglutaminase 2 Activation. Diabetes 62, 243–253 (2013).
Iismaa, S.E., Mearns, B.M., Lorand, L. & Graham, R.M. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol. Rev. 89, 991–1023 (2009).
Lee, Y.J., Jung, S.H., Kim, S.H., Kim, M.S., Lee, S., Hwang, J., Kim, S.Y., Kim, Y.M. & Ha, K.S. Essential Role of Transglutaminase 2 in Vascular Endothelial Growth Factor-Induced Vascular Leakage in the Retina of Diabetic Mice. Diabetes 65, 2414–2428 (2016).
Jung, S.H., Kong, D.H., Jeon, H.Y., Ji, S.H., Han, E.T., Park, W.S., Hong, S.H., Kim, M.S., Kim, Y.M. & Ha, K.S. Identification of transglutaminase 2 kinase substrates using a novel on-chip activity assay. Biosens. Bioelectron. 82, 40–48 (2016).
Mishra, S. & Murphy, L.J. Tissue transglutaminase has intrinsic kinase activity - Identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J. Biol. Chem. 279, 23863–23868 (2004).
Mishra, S. & Murphy, L.J. The p53 oncoprotein is a substrate for tissue transglutaminase kinase activity. Biochem. Biophys. Res. Commun. 339, 726–730 (2006).
Mishra, S., Saleh, A., Espino, P.S., Davie, J.R. & Murphy, L.J. Phosphorylation of histones by tissue transglutaminase. J. Biol. Chem. 281, 5532–5538 (2006).
Mishra, S., Melino, G. & Murphy, L.J. Transglutaminase 2 kinase activity facilitates protein kinase Ainduced phosphorylation of retinoblastoma protein. J. Biol. Chem. 282, 18108–18115 (2007).
Pietsch, M., Wodtke, R., Pietzsch, J. & Loser, R. Tissue transglutaminase: an emerging target for therapy and imaging. Bioorg. Med. Chem. Lett. 23, 6528–6543 (2013).
Siegel, M. & Khosla, C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol. Ther. 115, 232–245 (2007).
Jung, S.H., Jeon, H.Y., Lee, S.H., Han, E.T., Park, W.S., Hong, S.H., Kim, Y.M. & Ha, K.S. On-chip dual enzyme activity assay to investigate regulation of the transamidase and kinase activities of transglutaminase 2. Anal. Chim. Acta 1027, 92–100 (2018).
Liu, S., Cerione, R.A. & Clardy, J. Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc. Natl. Acad. Sci. U. S. A. 99, 2743–2747 (2002).
Pinkas, D.M., Strop, P., Brunger, A.T. & Khosla, C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 5, e327 (2007).
Lai, T.S. & Greenberg, C.S. TGM2 and implications for human disease: role of alternative splicing. Front. Biosci. Landmark Ed., 18, 504–519 (2013).
Jung, S.H., Kwon, M.H., Lee, S.H., Han, E.T., Park, W.S., Hong, S.H., Kim, Y.M. & Ha, K.S. Highthroughput investigation of transglutaminase 2 kinase regulation using a novel cysteine-modified peptide array. Anal. Biochem. 559, 62–70 (2018).
Jung, S.-H. & Ha, K.-S. Protein arrays for quantitative enzymatic profiling and serodiagnosis. Biochip J. 9, 269–277 (2015).
Lai, T.S., Davies, C. & Greenberg, C.S. Human tissue transglutaminase is inhibited by pharmacologic and chemical acetylation. Protein Sci. 19, 229–235 (2010).
Gundemir, S., Colak, G., Tucholski, J. & Johnson, G.V. Transglutaminase 2: a molecular Swiss army knife. Biochim. Biophys. Acta 1823, 406–419 (2012).
Kong, D.H., Jung, S.H., Jeon, H.Y., Kim, W.J., Kim, Y.M. & Ha, K.S. A peptide array-based serological protein kinase A activity assay and its application in cancer diagnosis. Analyst 140, 6588–6594 (2015).
Kim, S.H., Jung, S.H., Kong, D.H., Jeon, H.Y., Kim, M.S., Han, E.T., Park, W.S., Hong, S.H., Kim, Y.M. & Ha, K.S. Sensitive array-based assay for determination of serological protein kinase A autoantibody levels based on its antigen protein activation. Clin. Biochem. 49, 127–131 (2015).
Acknowledgements
This work was partially supported by grants from the National Research Foundation of Korea (2015R1A4A1038666 and 2016R1A2 A1A05004975 to KSH and 2107R1C1B1011535 to SHJ) and by a 2017 Research Grant from Kangwon National University (520170432 to KSH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interests
The authors declare no competing financial interests.
Conflict of Interests
Conflict of Interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Jung, SH., Kwon, MH., Han, ET. et al. Array-based Investigation of Amino Acids Responsible for Regulation of Transamidase and Kinase Activities of Transglutaminase 2. BioChip J 13, 251–259 (2019). https://doi.org/10.1007/s13206-019-3307-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13206-019-3307-3