Abstract
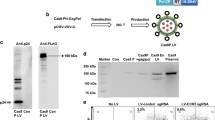
The CRISPR/Cas9 system is an effective tool for gene editing. However, this conventional expression system cannot control the timing of gene editing and does not utilize resistance screening markers. Therefore, carrying out CRISPR/Cas9 experiments is extremely inconvenient. Our aim is to develop an inducible lentiviral vector-based gene-editing system for C-X-C chemokine receptor 4 (CXCR4) by CRISPR/Cas9, and to demonstrate its function in MKN-45 cell. The DNA fragments of Blasticidin and T2A-GFP were produced using the lenti-Cas9-BLAST and PX458 plasmids as templates. The PCR products were harvested and cloned into the lenti-guide-puro plasmid to yield the lenti-guide-BLAST-GFP plasmid. Three double-stranded guide RNA (gRNA) sequences targeting the exon 2 of CXCR4 gene were designed online (http://crispr.mit.edu), synthesized, and recombined into the lenti-guide-BLAST-GFP plasmid, to yield the lenti-guide-BLAST-GFP-gRNA plasmid. The pCW-Cas9 and lenti-guide-BLAST-GFP-gRNA plasmids were packaged with lentiviral vectors, which were then transfected into MKN-45 cells, to identify the CXCR4 gene-editing effects using the T7 endonuclease 1 (T7E1) and Western blot assays. The lenti-guide-BLAST-GFP and lenti-guide-BLAST-GFP-gRNA plasmids were successfully constructed and packaged, to yield lentiviral particles. Transfection of the pCW-Cas9 and lenti-guide-BLAST-GFP-gRNA viral vectors could decrease the expression of CXCR4 protein, and lead to gene editing in MKN-45 cells. The efficiencies of gRNA-1, gRNA-2, and gRNA-3 were 45.6%, 53.6%, and 56.7%, respectively. Furthermore, the chemotactic efficiency of the dual viral vector-infected MKN-45 cells was significantly decreased in response to SDF-1. The numbers of migratory cells in the lower chamber of the transwell system were 30.0 ± 0.23, 29.7 ± 1.55, 28.2 ± 1.11 and 36.1 ± 2.00 cells per field (400×) for gRNA-1, gRNA-2, gRNA-3 and the control, respectively (P < 0.05). We constructed an inducible CXCR4 gene-editing, dual-vector CRISPR/Cas9 system, which could induce CXCR4 gene editing in MKN-45 cells in a doxycycline-dependent manner and thus reduce the migration of MKN-45 cells.





Similar content being viewed by others
Abbreviations
- CXCR4:
-
C-X-C chemokine receptor type 4
- SDF-1:
-
stromal cell-derived factor-1
- MKN-45:
-
gastric adenocarcinoma MKN-45 cells
- WHIM:
-
warts, hypogammaglobulinemia, infections, and myelokathexis syndrome
- ZFN:
-
zinc finger nuclease
- TALEN:
-
transcription activator-like effector nuclease
- CRISPR:
-
clustered regularly interspaced short palindromic repeats
- Cas9:
-
CRISPR-associated nuclease 9
- gRNA:
-
guide RNA
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- BLAST:
-
blasticidin
- GFP:
-
green fluorescent protein
References
Cao, J., Wu, L., Zhang, S. M., Lu, M., & Cheung, W. K. (2016). An easy and efficient inducible CRISPR/Cas9 platform with improved specificity for multiple gene targeting. Nucleic Acids Research, 44(19), e149.
Kawabata, K., Ujikawa, M., & Egawa, T. (1996). A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proceedings of the National Academy of Sciences of the United States of America, 96(10), 5663–7.
Liang, Y., Votaw, G., & Williams, S. (2005). Silencing of CXCR4 blocks breast cancer metastasis. Cancer Research, 65(3), 967–71.
Xu, C., Zhao, & Chen, Y. (2015). CXCR4 in breast cancer: oncogenic role and therapeutic targeting. Drug Design Development and Therapy, 9, 4953–64.
Hayasaka, K., Yoshimura, D., Nakayama, H., Shioda, E. E., & Miya- saka, T. (2015). The HIV-1 Gp120/CXCR4 axis promotes CCR7 ligand-dependent CD4 T cell migration: CCR7 homo- and CCR7/CXCR4 hetero-oligomer formation as a possible mechanism for up-regulation of functional CCR7. PLoS ONE, 10(2), e0117454.
Hou, C., Wang, S., Yu, X., & Chen, S. (2015). Genome editing of CXCR4 by CRISPR/cas9 confers cells resistant to HIV-1 infection. Scientific Reports, 5, 15577.
Guyon, A. (2014). CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Frontiers in Cellular Neuroscience, 8, 65.
Hernandez, P. A., Gorlin, R. J., Lukens, J. N., Taniuchi, S., & Bohinjec, J. (2003). Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nature Genetics, 34(1), 70–4.
McDermott, D. H., Gao, J. L., Siwicki, M., & Martens, C. (2015). Chromothriptic cure of WHIM syndrome. Cell, 160(4), 686–99.
Shao, M., Xu, T. R., & Chen, C. S. (2016). The big bang of genome editing technology: development and application of the CRISPR/Cas9 system in disease animal models. Zoological Research, 37(4), 191–204.
Gaj, T., Gersbach, D. A., & Barbas, C. F. (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends in Biotechnology, 31(7), 397–405.
Ding, Y., Li, H., Chen, L.L., & Xie. (2016). Recent advances in genome editing using CRISPR/Cas9. Frontiers in Plant Science, 7, 703.
Huo, W., Zhao, G., Yin, J., Ouyang, X., & Wang, Y. (2017). Lentiviral CRISPR/Cas9 vector mediated miR-21 gene editing inhibits the epithelial to mesenchymal transition in ovarian cancer cells. Journal of Cancer, 8(1), 57–64.
de Solis, C. A., Ho, A., & Holehonnur, R. (2016). The development of a viral mediated CRISPR/Cas9 system with doxycycline dependent gRNA expression for inducible in vitro and in vivo genome editing. Front Mol Neurosci, 9, 70.
Karginov, F. V., & Hannon, G. J. (2010). The CRISPR system: small RNA- guided defense in bacteria and archaea. Molecular Cell, 37(1), 7–19.
Marraffini, L. A., & Sontheimer, E. J. (2010). CRISPR interference: RNA-directed a- daptive immunity in bacteria and archaea. Nature Reviews Genetics, 11(3), 181–90.
Mojica, F. J., Díez-Villaseñor, C., Soria, E., & Juez, G. (2000). Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria. Molecular Microbiology, 36, 244–6.
Schambach, A., Zychlinski, D., Ehrnstroem, B., & Baum, C. (2013). Biosafety features of lentiviral vectors. Human Gene Therapy, 24(2), 132–42.
Durand, S., & Cimarelli, A. (2011). The inside out of lentiviral vectors. Viruses, 3(2), 132–59.
Dull, Z., Kelly, M., & Nguyen, M. (1998). A third-generation lentivirus vector with a conditional packaging system. Journal of Virology, 72(11), 8463–71.
Mieno, S., Ramlawi, B., Boodhwani, M., Clements, R. T., & Minamimura, K. (2006). Role of stromal-derived factor-1alpha in the induction of circulating CD34+CXCR4+ progenitor cells after cardiac surgery. Circulation, 114, I186–92.
Nakano, H., Lyons-Cohen, M. R., Whitehead, S. S., Nakano, K., & Cook, D. N. (2017). Distinct functions of CXCR4, CCR2, and CX3CR1 direct dendritic cell precursors from the bone marrow to the lung. Journal of Leukocyte Biology, 101(5), 1143–53.
Ratajczak, M. Z., Zuba-Surma, E., Kucia, M., Reca, R., Wojakowski, W., & Ratajczak, J. (2006). The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia, 20(11), 1915–24.
Murdoch, C. (2000). CXCR4: chemokine receptor extraordinaire. Immunological Reviews, 177, 175–84.
Heitz, F., Johansson, T., Baumgärtel, K., Gecaj, R., Pelczar, P., & Mansuy, I. M. (2014). Heritable and inducible gene knockdown in astrocytes or neurons in vivo by a combined lentiviral and RNAi approach. Frontiers in Cellular Neuroscience, 8, 62.
Zetsche, B., Volz, S. E., & Zhang, F. (2015). A split-Cas9 architecture for inducible genome editing and transcription modulation. Nature Biotechnology, 33(2), 139–42.
Wang, T., Wei, J. J., Sabatini, D. M., & Lander, E. S. (2014). Genetic screens in human cells using the CRISPR-Cas9 system. Science, 343(6166), 80–4.
Hsu, P. D., Scott, D. A., Weinstein, J. A., Ran, F. A., & Konermann, S. (2013). DNA targeting specificity of RNA-guided Cas9 nucleases. Nature Biotechnology, 31(9), 827–32.
Acknowledgements
The authors would like to thank the staff of the Chongqing Key Laboratory of Pediatrics for their valuable contributions to this project.
Funding
This work was financially supported by the National Natural Science Foundation of China [No. 30700785] and the Chongqing Health and Family Planning Commission [No. 2015MSXM040]. The funders had no role in the design of the study, data collection and analysis, decision for publication, or preparation of the manuscript.
Author Contributions
Y.P. and S.W. carried out the molecular laboratory work, participated in the data analysis, carried out the sequence alignments, participated in the design of the study and drafted the manuscript; T.Y. carried out the statistical analyses; and X.T. and F.C. conceived, designed, and coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Peng, Y., Yang, T., Tang, X. et al. Construction of an Inducible CRISPR/Cas9 System for CXCR4 Gene and Demonstration of its Effects on MKN-45 Cells. Cell Biochem Biophys 78, 23–30 (2020). https://doi.org/10.1007/s12013-019-00898-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-019-00898-x