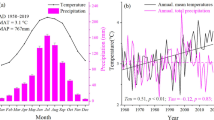
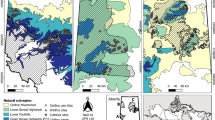
Abstract
Lianas have a huge influence on forest structure and function. However, it is unclear how the surrounding environment affects the establishment of liana seedlings in temperate forests. We addressed the following questions: (1) Can current-year seedlings persist under a closed canopy? (2) Do current-year seedlings form aggregated distribution and how has their spatial distribution varied over the years? (3) How does the light condition, soil moisture content, forest floor litter, understory vegetation, and the distance from the conspecific adults affect the establishment and survival of seedlings? We examined the distribution pattern and survivorship of current-year seedlings of the temperate liana species, Wisteria floribunda, across a heterogeneous environment for 6 years using 1 m2 sub-quadrats (n = 651) in a 6 ha plot within the Ogawa Forest Reserve, an old-growth, temperate, deciduous forest in central Japan. In total, 908 current-year seedlings were observed during the study period, 87% of which emerged in 2014. Over half (56%) of these seedlings survived until 1 year after germination, which was relatively high compared with other tree species in this forest. The seedlings formed significantly aggregated distribution, but the degree of aggregation decreased over time. The number of emerged seedlings was negatively associated with the presence of dwarf bamboo (Sasa borealis) and the distance from the nearest conspecific adult. However, the survival rate of the seedlings was negatively associated with the presence of dwarf bamboo and soil moisture content and was positively associated with the openness of the canopy and the distance from the nearest conspecific adult. An enhanced survival rate under more intense light conditions and the ability to persist within the shaded understory may be important for the survival of this species in the earlier stage of the life history.



Similar content being viewed by others
References
Abe S, Masaki T, Nakashizuka T (1995) Factors influencing sapling composition in canopy gaps of a temperate deciduous forest. Vegetatio 120:21–32
Abe M, Izaki J, Miguchi H, Masaki T, Makita A, Nakashizuka T (2002) The effects of Sasa and canopy gap formation on tree regeneration in an old beech forest. J Veget Sci 13:565–574
Allen BP, Pauley EF, Sharitz RR (1997) Hurricane impacts on liana populations in an old-growth southeastern bottomland forest. J Torrey Bot Soc 124:34–42
Boyden S, Binkley D, Shepperd W (2005) Spatial and temporal patterns in structure, regeneration, and mortality of an old-growth ponderosa pine forest in the Colorado Front Range. Forest Ecol Manag 219:43–55
Bürkner PC (2017) brms: an R package for Bayesian multilevel models using Stan. J Stat Softw 80:1–28
Carrasco-Urra F, Gianoli E (2009) Abundance of climbing plants in a southern temperate rain forest: host tree characteristics or light availability? J Veget Sci 20:1155–1162
Clark DA, Clark DB (1984) Spacing dynamics of a tropical rain forest tree: evaluation of the Janzen–Connell model. Am Nat 124:769–788
Clark JS, Silman M, Kern R, Macklin E, HilleRisLambers J (1999) Seed dispersal near and far: patterns across temperate and tropical forests. Ecology 80:1475–1494
Clover G, Denton J, Denton G (2015) First report of Wisteria vein mosaic virus on Wisteria spp. in the United Kingdom. New Dis Rep 31:2044–2058
Comita LS, Queenborough SA et al (2014) Testing predictions of the Janzen–Connell hypothesis: a meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. J Ecol 102:845–856
Ellsworth JW, Harrington RA, Fownes JH (2004a) Survival, growth and gas exchange of Celastrus orbiculatus seedlings in sun and shade. Am Midl Nat 151:233–240
Ellsworth JW, Harrington RA, Fownes JH (2004b) Seedling emergence, growth, and allocation of oriental bittersweet: effects of seed input, seed bank, and forest floor litter. For Ecol Manag 190:255–264
Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB (2014) Bayesian data analysis, vol 2. CRC Press, Boca Raton
George B, Seals S, Aban I (2014) Survival analysis and regression models. J Nucl Cardiol 21:686–694
Gianoli E, Saldaña A, Jiménez-Castillo M, Valladares F (2010) Distribution and abundance of vines along the light gradient in a southern temperate rain forest. J Veget Sci 21:66–73
Greenberg CH, Smith LM, Levey DJ (2001) Fruit fate, seed germination and growth of an invasive vine—an experimental test of “sit and wait” strategy. Biol Invasions 3:363–372
Iida S (2004) Indirect negative influence of dwarf bamboo on survival of Quercus acorn by hoarding behavior of wood mice. For Ecol Manag 202:257–263
Ito H, Hino T (2004) Effects of deer, mice and dwarf bamboo on the emergence, survival and growth of Abies homolepis (Piceaceae) seedlings. Ecol Res 19:217–223
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Joseph M (2016) Exact sparse car models in stan. Stan Official Website. https://mc-stan.org/users/documentation/case-studies/mbjoseph-CARStan.html. Accessed 31 Dec 2019
Ladwig LM, Meiners SJ (2015) The role of lianas in temperate tree communities. In: Schnitzer SA, Bongers F, Burnham RJ, Putz FE (eds) Ecology of lianas. Wiley, Oxford, pp 188–202
Lan G, Zhu H et al (2009) Spatial dispersion patterns of trees in a tropical rainforest in Xishuangbanna, southwest China. Ecol Res 24:1117–1124
Law R, Illian J, Burslem DFRP, Gratzer G, Gunatilleke CVS, Gunatilleke IAUN (2009) Ecological information from spatial patterns of plants: insights from point process theory. J Ecol 97:616–628
Ledo A, Schnitzer SA (2014) Disturbance and clonal reproduction determine liana distribution and maintain liana diversity in a tropical forest. Ecology 95:2169–2178
Leicht SA, Silander JA (2006) Differential responses of invasive Celastrus orbiculatus (Celastraceae) and native C. scandens to changes in light quality. Am J Bot 93:972–977
Leicht-Young SA, Silander JA Jr, Latimer AM (2007) Comparative performance of invasive and native Celastrus species across environmental gradients. Oecologia 154:273–282
Leicht-Young SA, Pavlovic NB, Frohnapple KJ, Grundel R (2010) Liana habitat and host preferences in northern temperate forests. For Ecol Manag 260:1467–1477
Leicht-Young SA, Pavlovic NB, Grundel R (2013) Susceptibility of eastern US habitats to invasion of Celastrus orbiculatus (oriental bittersweet) following fire. For Ecol Manag 302:85–96
Li HH, Nishimura H, Hasegawa K, Mizutani J (1992) Allelopathy of Sasa cernua. J Chem Ecol 18:1785–1796
Londré RA, Schnitzer SA (2006) The distribution of lianas and their change in abundance in temperate forests over the past 45 years. Ecology 87:2973–2978
Manzané-Pinzón E, Goldstein G, Schnitzer SA (2018) Does soil moisture availability explain liana seedling distribution across a tropical rainfall gradient? Biotropica 50:215–224
Masaki T, Nakashizuka T (2002) Seedling demography of Swida controversa: effect of light and distance to conspecifics. Ecology 83:3497–3507
Masaki T, Suzuki W, Niiyama K, Iida S, Tanaka H, Nakashizuka T (1992) Community structure of a species-rich temperate forest, Ogawa Forest Reserve, central Japan. Vegetatio 98:97–111
Masaki T, Tanaka H, Tanouchi H, Sakai T, Nakashizuka T (1999) Structure, dynamics and disturbance regime of temperate broad-leaved forests in Japan. J Veget Sci 10:805–814
Masaki T, Hata S, Ide Y (2015) Heterogeneity in soil water and light environments and dispersal limitation: what facilitates tree species coexistence in a temperate forest? Plant Biol 17:449–458
Masaki T, Nakashizuka T, Niiyama K, Tanaka H, Iida S, Bullock JM, Naoe S (2019) Impact of the spatial uncertainty of seed dispersal on tree colonization dynamics in a temperate forest. Oikos. https://doi.org/10.1111/oik.06236
Mizoguchi Y, Morisawa T, Ohtani Y (2002) Climate in Ogawa Forest Reserve. In: Nakashizuka T, Matsumoto Y (eds) Diversity and interaction in a temperate forest community: Ogawa Forest Reserve of Japan. Springer Japan, Tokyo, pp 11–18
Mori H, Kamijo T, Masaki T (2016) Liana distribution and community structure in an old-growth temperate forest: the relative importance of past disturbances, host trees, and microsite characteristics. Plant Ecol 217:1171–1182
Mori H, Ueno S, Matsumoto A, Kamijo T, Tsumura Y, Masaki T (2017) Large contribution of clonal reproduction to the distribution of deciduous liana species (Wisteria floribunda) in an old-growth cool temperate forest: evidence from genetic analysis. Ann Bot 121:336–359
Muller-Landau HC, Wright SJ, Calderón O, Condit R, Hubbell SP (2008) Interspecific variation in primary seed dispersal in a tropical forest. J Ecol 96:653–667
Nakashizuka T (1988) Regeneration of beech (Fagus crenata) after the simultaneous death of undergrowing dwarf bamboo (Sasa kurilensis). Ecol Res 3:21–35
Nakashizuka T (2002) Disturbance regimes. In: Matsumoto Y, Nakashizuka T (eds) Diversity and interaction in a temperate forest community: Ogawa Forest Reserve of Japan. Springer Japan, Tokyo, pp 67–80
Nakashizuka T, Matsumoto Y (2002) Diversity and interaction in a temperate forest community: Ogawa Forest Reserve of Japan. Springer Japan, Tokyo
Nakashizuka T, Iida S, Tanaka H, Shibata M, Abe S, Masaki T, Niiyama K (1992) Community dynamics of Ogawa Forest Reserve, a species rich deciduous forest, central Japan. Vegetatio 103:105–112
Nakashizuka T, Iida S, Masaki T, Shibata M, Tanaka H (1995a) Evaluating increased fitness through dispersal: a comparative study on tree populations in a temperate forest, Japan. Ecoscience 2:245–251
Nakashizuka T, Katsuki T, Tanaka H (1995b) Forest canopy structure analyzed by using aerial photographs. Ecol Res 10:13–18
Nakayama S, Inokuchi M, Minamitani T (2004) Seeds of wild plants in Japan. Tohoku University Press, Sendai
Naoe S, Masaki T, Sakai S (2018) Effects of temporal variation in community-level fruit abundance on seed dispersal by birds across woody species. Am J Bot 105:1792–1801
Niiyama K, Abe S (2002) Tree demography throughout the tree life cycle. In: Nakashizuka T, Matsumoto Y (eds) Diversity and interaction in a temperate forest community: Ogawa Forest Reserve of Japan. Springer Japan, Tokyo, pp 155–166
Ohashi H (1989) Wisteria. In: Satake Y, Hara H, Watari S, Tominari T (eds) Wild flowers of Japan: Woody plants. Heibonsha, Tokyo, pp 247–248 (in Japanese)
Padaki A, Parthasarathy N (2000) Abundance and distribution of lianas in tropical lowland evergreen forest of agumbe, central western ghats, india. Trop Ecol 41:143–154
Pélissier R, Goreaud F (2015) Ads package for R: a fast unbiased implementation of the K-function family for studying spatial point patterns in irregular-shaped sampling windows. J Stat Softw 63:1–18
Perry GLW, Miller BP, Enright NJ (2006) A comparison of methods for the statistical analysis of spatial point patterns in plant ecology. Plant Ecol 187:59–82
Putz FE (1984) How trees avoid and shed lianas. Biotropica 16:19–23
QGIS Development Team (2009) QGIS geographic information system. Open Source Geospatial Foundation Project. http://qgis.osgeo.org
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2016
Roiloa SR, Sánchez-Rodríguez P, Retuerto R (2014) Heterogeneous distribution of soil nutrients increase intra-specific competition in the clonal plant Glechoma hederacea. Plant Ecol 215:863–873
Sakai A, Nomiya H, Suzuki W (2002) Horizontal distribution of stolons of a temperate liana Wisteria floribunda DC and its ecological significance. J For Res 7:125–130
Schnitzer SA (2018) Testing ecological theory with lianas. New Phytol 220:366–380
Schnitzer SA, Bongers F (2002) The ecology of lianas and their role in forests. Trends Ecol Evol 17:223–230
Schnitzer SA, Bongers F (2011) Increasing liana abundance and biomass in tropical forests: emerging patterns and putative mechanisms. Ecol Lett 14:397–406
Schnitzer SA, Dalling JW, Carson WP (2000) The impact of lianas on tree regeneration in tropical forest canopy gaps: evidence for an alternative pathway of gap-phase regeneration. J Ecol 88:655–666
Schnitzer SA, Mangan SA et al (2012) Liana abundance, diversity, and distribution on Barro Colorado Island, Panama. PLoS One 7:e52114
Shibata M, Masaki T, Tanaka H, Niiyama K, Iida S, Abe S, Nakashizuka T (2010) Effects of abiotic and biotic factors and stochasticity on tree regeneration in a temperate forest community. Écoscience 17:137–145
Stan Development Team (2016) RStan: the R interface to Stan. R package version 2.14.1. http://mc-stan.org
Tadokoro K, Yajima T (1990) Growth and activity of rhizomes of Sasa nipponica. J Jpn For Soc 72:345–348 (In Japanese)
Tanaka H, Nakashizuka T (1997) Fifteen years of canopy dynamics analyzed by aerial photographs in a temperate deciduous forest, japan. Ecology 78:612–620
Valladares F, Gianoli E, Saldana A (2011) Climbing plants in a temperate rainforest understorey: searching for high light or coping with deep shade? Ann Bot 108:231–239
Varvaro L (1987) A bacterial leaf spot of wisteria in Italy. EPPO Bull 17:287–290
Walters MB, Reich PB (2000) Seed size, nitrogen supply, and growth rate affect tree seedling survival in deep shade. Ecology 81:1887–1901
Wenger SJ, Freeman MC (2008) Estimating species occurrence, abundance, and detection probability using zero-inflated distributions. Ecology 89:2953–2959
Yamazaki K, Sugiura S (2008) Arthropods associated with bacterium galls on wisteria. Appl Entomol Zool 43:191–196
Yoshinaga S, Takahashi M, Aizawa S (2002) Landforms and soil characteristics in Ogawa Forest Reserve. In: Nakashizuka T, Matsumoto Y (eds) Diversity and interaction in a temperate forest community: Ogawa Forest Reserve of Japan. Springer Japan, Tokyo, pp 19–26
Acknowledgements
We would like to thank Drs. Takashi Kamijo, Yoshihiko Tsumura, Tatsuyuki Seino, and Kiyokazu Kawada for their helpful comments on this study, Dr. James Worth for revising the manuscript, and Drs. Yayoi Takeuchi, Shota Sakaguchi, and Mr. Shota Harasawa for field assistance. This study was supported by a Grant-in-Aid for JSPS Research Fellow from the Japan Society for the Promotion of Science (Grant No. 16J00768). This study was partly supported by Monitoring Sites 1000, Ministry of Environment, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mori, H., Masaki, T., Tsunamoto, Y. et al. Survival rate and environmental response of current-year seedlings of the temperate liana Wisteria floribunda across a heterogeneous environment. J Plant Res 133, 193–203 (2020). https://doi.org/10.1007/s10265-019-01163-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10265-019-01163-1