Abstract
Ethylene glycol dry reforming (EGDR) was investigated for the first time on 10% Co/Al2O3 and 3% Ce–10% Co/Al2O3 catalysts at stoichiometric feed composition under atmospheric pressure and 923–998 K for syngas production. Catalysts were characterized using BET, H2-TPR, XRD and Raman spectroscopy measurements. The addition of Ce promoter eased the reduction of Co3O4 with lower reduction temperature and enhanced metal dispersion. Ce promotion also improved EGDR performance by increasing reactant conversions, syngas yields and reducing undesirable methane formation. The conversion of ethylene glycol and H2 yield reached up to 71.7% and 69.3%, respectively.
Similar content being viewed by others
Introduction
The extensive use of fossil fuels for transportation and industrial production has led to severe environmental pollution related to CO2 greenhouse gas emissions [1]. At the same time, global oil reserves are depleting rapidly and hence, there is a dire need to find new alternative and sustainable energy sources. Considerable attention has been dedicated to search for renewable energies to partially replace petroleum-based resources. The use of bio-renewable organic sources including biomass-derived oxygenated compounds as feedstocks in catalytic reforming processes to yield syngas for synthetic fuel production has emerged as a promising solution [2, 3]. Syngas is a mixture of H2 and CO which acts as building block for Fischer–Tropsch synthesis (FTS) to produce long-chain hydrocarbons followed by refinery to generate synthetic fuels and valuable petrochemicals [4, 5].
Amongst the oxygenated compounds, ethylene glycol (EG) emerges as an alluring feedstock for syngas generation because it is the simplest polyol, major bio-oil constituent and can be derived from cellulose and sugar [6,7,8]. At present, there are two existing EG reforming techniques for syngas generation, namely, aqueous phase reforming and steam reforming. The aqueous phase reforming is normally conducted at moderate temperature and high pressure (explicitly, T = 498–723 K and P = 2.9–25 MPa) [9], whereas steam reforming of EG is conventionally operated at atmospheric pressure. However, these current reforming techniques consume significant quantity of water and release excessive amounts of undesirable CO2 to environment (cf. Eq. 1):
In this regard, ethylene glycol dry reforming, EGDR [see Eq. (2)] appears as an attractive and green reforming process, since it consumes CO2 greenhouse gas and biomass-derived EG to generate syngas with a low H2/CO ratio suitable for FTS [4, 5]. Although aqueous phase reforming and steam reforming processes of ethylene glycol were substantially inspected on diverse catalysts for syngas production, EGDR process has not been investigated before in literature. Thus, it is crucial to explore this novel technique to produce ecofriendly and desirable syngas from low-valued and unwanted reactants:
Cobalt-based catalysts have been widely implemented as catalyst for various reforming techniques (such as ethanol steam reforming [10], ethanol dry reforming [11], methane dry reforming [12, 13] and EG steam reforming [14, 15]) and exhibited outstanding performance. However, carbon deposition on catalyst surface is the common setback observed for cobalt catalyst [16]. Ceria promoter was recently reported to impede carbonaceous formation in reforming processes, since its high reducibility, oxygen mobility and oxygen storage capacity arising from Ce4+ ↔ Ce3+ redox cycle could gasify carbon deposits from catalyst surface [11, 17, 18]. Therefore, the goal of this work is to examine the feasibility of EGDR on Ce-promoted Co/Al2O3 catalysts at different reaction temperatures.
Experimental
Catalyst synthesis
10% Co/Al2O3 catalyst was prepared using an incipient wetness impregnation (IWI) approach. The pretreatment of γ-Al2O3 support (Sasol, Puralox SCCa-150/200) was done by calcination in flowing air at 1023 K for 5 h to avoid structural alteration during catalytic tests. Then, the calcined γ-Al2O3 support was blended with an accurately balanced amount of Co(NO3)2.6H2O (Sigma-Aldrich) aqueous solution in a BÜCHI Rotavapor R-200 rotary evaporator for 2 h. The soaked solid was dried overnight at 333 K in an oven and calcined in air for 5 h at 1023 K (ramping rate 5 K min−1). The sequential IWI method was implemented for preparing 3% Ce–10% Co/Al2O3. In particular, 10% Co/Al2O3 was further impregnated with Ce(NO3)3.6H2O (Merck Millipore) as promoter precursor using the aforesaid IWI conditions. Both catalysts were crushed in mortar using pestle followed by sieving to desired particle size (125–160 μm).
Catalyst characterization
Brunauer–Emmett–Teller (BET) surface area, total pore volume and pore diameter were determined in a Micromeritics TriStar II 3020 V1.04 instrument at 77 K. All samples were outgassed at 573 K for 1 h in N2 flow prior to measurements to eliminate traced moisture and foreign adsorbed molecules. H2 temperature-programmed reduction (H2-TPR) was achieved from a Micromeritics AutoChem II-2920 chemisorption system. Catalyst (roughly 0.1 g) placed in a U-tube (quartz) by quartz wool was pretreated at 373 K for 30 min in He gas (50 ml min−1) before being reduced in 10%H2/Ar (50 ml min−1) from 373 to 1173 K with a linear heating rate (10 K min−1). Specimen was retained at final temperature for 30 min and then quenched to ambient temperature.
The crystal structure of fresh calcined, reduced and spent catalysts was examined in a Rigaku Miniflex II X-ray powder diffraction (XRD) system employing Cu as the radiation source. This unit was operated at 30 kW and 15 mA with wavelength, λ of 1.5418 Å; whereas diffraction patterns were verified from 2θ = 3° to 80° with small scan speed and step size of 1 min−1 and 0.02° in that order to yield a high resolution. The JASCO NRS-3100 system was employed for Raman analyses of spent catalysts. A 532-nm green laser with laser power less than 5 mW was used during Raman measurements.
Temperature-programmed oxidation (TPO) was performed on TGA Q500 thermogravimetric unit (TA Instruments) for the precise quantification of deposited carbon on used catalysts. After being treated with flowing N2 gas (100 ml min−1) for 0.5 h at 373 K to remove moisture, 20% O2/N2 oxidizing mixture was purged through spent catalysts with temperature increment from 373 to 1023 K (10 K min−1) for the successful oxidation of carbonaceous species. Afterward, the final temperature was kept constant for 30 min in the same gaseous mixture.
Ethylene glycol dry reforming experiments
EGDR tests were done at atmospheric pressure, stoichiometric feed ratio (\({\text{Co}}_{3} O_{4} + H_{2} \to 3CoO + H_{2} O\)) and 923–998 K in a standard stainless-steel fixed-bed continuous flow reactor for 8 h with total flow rate of 70 ml min−1. Quartz wool was used to hold around 0.1 g of catalyst in the middle of the reactor. Catalyst was activated in 50% H2/N2 (60 ml min−1) at 1023 K for 2 h and then purged in N2 flow for 30 min prior to reaction. After being pumped by a precise KellyMed KL-602 syringe pump through a pre-heater, vaporised EG was fed to the top of reactor where it was mixed with CO2 reactant and N2 diluent (regulated by Alicat mass flow controllers). Gas hourly space velocity was kept at 42 l g −1cat h−1 during each run. The composition of gaseous outlet from reactor was determined in Agilent 6890 Series GC system using TCD detector. N2 internal standard was continuously measured with time on stream to assure the high accuracy of GC analysis and experimental works. The error of nitrogen balance was less than 3.76% for all runs, whilst the relative error among repeated experiments at similar conditions was smaller than 6.32%.
EGDR reaction metrics including reactant conversion (Xi; i: C2H6O2 or CO2) and gaseous product yield, Y are given in Eqs. (3)–(6). The conversion of EG into gaseous products was estimated based on atomic H balance as shown in Eq. (3); whereas \(F^{\text{in}}\) and \(F^{\text{out}}\) refer to inlet and outlet molar flow rates of the corresponding components:
Results and discussion
Textural properties of catalysts
The physical features of fresh 10% Co/Al2O3 and 3% Ce–10% Co/Al2O3 are summarized in Table 1. A minor drop in BET surface area with Ce addition from 142.9 to 134.7 m2 g−1 (about 5.7%) was observed; whereas the total pore volume of both catalysts seemed to be equivalent. These results could be indicative of uniform dispersion of Ce promoter on catalyst surface.
H2 temperature-programmed reduction
Figure 1 shows the reduction behavior of both catalysts during H2-TPR. Three deconvoluted H2 consumption peaks are observed and labeled as P1, P2 and P3. The H2-reduction of Co3O4 phase was broadly regarded as a two-step procedure in which Co3O4 was firstly reduced to CoO phase [cf. Eq. (7)] followed by reduction to metallic Co0 [cf. Eq. (8)] [11, 20]. As a result, low-temperature peaks P1 and P2 ranging from 650 to 900 K were ascribed to the reduction of Co3O4 to CoO and CoO to Co0, respectively.
Remarkably, as seen in Fig. 1, both peaks P1 and P2 of Ce-doped catalyst shifted towards lower temperature region in comparison with 10% Co/Al2O3. The addition of CeO2 promoter facilitated Co3O4 reduction plausibly because of extra electron density on Co3O4 donated by CeO2, thereby relieving reduction procedure [11]. The formation of the third peak, P3 at temperature above 900 K for both samples corresponds to the reduction of spinel CoAl2O4 [see Eq. (9)], normally formed due to diffusion of Co ions into the vacancies in lattice sites of Al2O3 during calcination at high temperature [21]. Since the trivalent Co3+ and Al3+ ions have similar ionic radii of 0.063 and 0.054 nm, respectively, Co3+ ions in Co3O4 could be substituted by Al3+ ions to yield CoAl2O4 phase having strong metal–support interaction, thereby inducing high reduction temperature [22]. Notably, the reduction temperature for peak P3 of 3% Ce–10% Co/Al2O3 was superior to that of 10% Co/Al2O3 as seen in Fig. 1. It could be caused by double calcination during sequential IWI and hence a greater amount of CoAl2O4 could be yielded with a stronger degree of metal–support interaction:
X-ray diffraction measurements
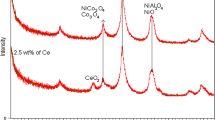
Figure 2 presents the XRD patterns of fresh calcined and reduced catalysts. The X-ray diffractograms of calcined γ-Al2O3 support is provided to ease the peak assignments. All XRD patterns were examined with the employment of database library from Joint Committee on Powder Diffraction Standards (JCPDS) [23]. All catalysts show five characteristic peaks belonging to γ-alumina at 2θ = 18.9°, 32.5°, 37.0°, 45.6° and 67.1° (JCPDS No. 04-0858) [11, 24]. Cubic spinel Co3O4 phase (2θ located at 31.2°, 37.0°, 45.0° and 55.7°) and CoAl2O4 form (2θ = 59.4° and 65.3°) were found on both calcined promoted and unpromoted samples (Fig. 2b, c) based on JCPDS No. 74-2120 and JCPDS No. 82-2246, respectively [23]. The presence of Co3O4 and CoAl2O4 phases is consistent with peak assignment in H2-TPR measurement (see Fig. 1). For the calcined promoted catalyst, CeO2 phase was detected at 2θ = 28.8° (see Fig. 2c) according to JCPDS No. 34-0394 [18]. The CeO2 form was most likely produced from the oxidation of Ce2O3 previously yielded by Ce(NO3)3 thermal decomposition. For the reduced catalysts (Fig. 2d, e), the absence of CoAl2O4 phase further reinforces the complete H2 reduction of CoAl2O4 form [see Eq. (9)] in agreement with H2-TPR (cf. Fig. 1). The Co0 metallic phase appearing in the XRD patterns of both reduced catalysts at 2θ diffractive peak about 51.5° (JCPDS No. 15-0806) was assigned to Co3O4 → Co reduction in H2 activation [23]. Based on H2-TPR results (Fig. 1), Co3O4 was reduced thoroughly to final Co0 form at below 1000 K. Thus, the co-presence of Co3O4 phase on reduced catalysts was because of inevitable Co re-oxidation in air during ex situ XRD measurements.
The average crystallite size of Co3O4 phase for both catalysts was estimated using the Scherrer equation for the highest peak (2θ = 31.2°) [19]. As listed in Table 1, Ce promotion reduced substantially Co3O4 crystallite size from 21.4 to 9.2 nm. The tiny crystallite size of Ce-promoted catalyst less than 10 nm could indicate the fine distribution of Co3O4 on support surface. The enhancing metal dispersion for promoted catalyst was possibly attributed to the dilution effect in which CeO2 promoter could dilute Co3O4 particles, thereby preventing them from agglomeration.
Ethylene glycol dry reforming evaluation
Figure 3 shows the influence of temperature varied from 923 to 998 K on C2H6O2 and CO2 conversions at stoichiometric feedstock. Regardless of specimens, reactant conversions substantially increased with rising reaction temperature. This could be attributed to the endothermic nature of EGDR reaction. In particular, EG conversion grew from 59.0 to 71.7% with rising temperature from 923 to 998 K on 3% Ce–10% Co/Al2O3; whereas CO2 conversion enhanced from 38.3 to 48.7%. As seen in Fig. 3, irrespective of catalysts, EG conversion was greater than CO2 conversion for all temperatures. This observation could indicate the presence of concomitant EG steam reforming side reaction [cf. Eq. (1)] during EGDR. This side reaction consumes EG but yields undesirable CO2 by-product and, hence, lessening CO2 conversion.
Notably, the overall catalytic activity was improved with Ce modification for all temperatures in terms of CO2 and EG conversions as shown in Fig. 3. The enhancement of reactant conversions could be assigned to the basic property of CeO2 promoter which could increase CO2 adsorption on catalyst surface. The high oxygen storage and release capacity of CeO2 also improve catalytic activity, since it could induce carbonaceous species gasification during EGDR, thereby maintaining the virgin catalyst surface [11, 25]. In addition, the smaller Co3O4 crystallite size of 3% Ce–10% Co/Al2O3 (see Table 1) due to high active metal dispersion could greatly contribute to the increasing reactant conversions.
The impact of Ce-dopant and temperature towards H2 and CO yields of 10% Co/Al2O3 is displayed in Fig. 4. The relationship between temperature and yield of H2 and CO also follows the similar trend of temperature vs. reactant conversions (see Fig. 3). In general, H2 and CO yields increased with increasing temperature from 923 to 998 K and reached up to 69.3% and 70.8% in this order. The rising syngas yield further confirms the enhancement of EGDR reaction with temperature.
Interestingly, CH4 by-product was also observed during EGDR (see Fig. 5). It could be formed from incomplete EG decomposition as given in Eq. (10). However, the amount of CH4 yield seemed to be negligible (< 2.5%) for both samples and lessened with rising temperature from 923 to 998 K. The decreasing trend in the yield of CH4 intermediate product with temperature could be due to the occurrence of secondary reactions such as dry reforming of methane [cf. Eq. (11)] and steam reforming of methane [cf. Eq. (12)]. The significantly low CH4 yield is indicative of successful reforming of EG to final valuable syngas:
Figure 6 illustrates the influence of temperature on H2/CO ratio for both catalysts. Generally, regardless of catalysts, H2/CO ratio decreases with increasing temperature as a result of thermodynamically preferred reverse water–gas shift (RWGS) and reverse Boudouard reactions [see Eqs. (13) and (14), respectively] concomitantly occurring during EGDR. However, the H2/CO ratios are still higher compared to the stoichiometric H2/CO value of 0.67 for EGDR [cf. Eq. (2)]. This phenomenon could be a result of EG steam reforming reaction [see Eq. (1)] between H2O (formed from EGDR) and EG to yield extra H2 product:
Figure 7 displays the conversion envelopes of EG and CO2 reactants with time on stream. Irrespective of time on stream, EG and CO2 conversions on Ce-doped catalyst are greater than those of unpromoted counterpart. For unpromoted catalyst, EG and CO2 conversions declined substantially during 8 h with deactivation degree of 13.38% and 25.78%, respectively (see Table 2); whereas, a slight decrease in catalytic activity was observed for 3% Ce–10% Co/Al2O3. In fact, the smaller degree of deactivation of 4.45% and 7.13% was evidenced for the corresponding EG and CO2 conversions on 3% Ce–10% Co/Al2O3. The enhanced catalytic stability with Ce addition was owing to the high oxygen mobility and redox property of CeO2 promoter easing carbon removal during EGDR [11, 25].
Characterization of spent catalysts
The crystalline structure of spent catalysts after EGDR at stoichiometric feed composition and T = 998 K was examined by XRD measurements and shown in Fig. 8. Both catalysts possessed broad peaks with high intensity at 2θ = 26.4° corresponding to graphitic carbon (JCPDS No. 75-0444) [26, 27]. The presence of graphite on catalyst surface was inevitable, since EGDR was conducted at high reaction temperature (≥ 923 K) and deposited carbon was formed from EG decomposition. Co3O4 phase was also detected for both spent samples as a result of metallic Co0 oxidation when spent catalysts were exposed to air during catalyst discharge. However, the characteristic peak for CeO2 was not identified for spent 3% Ce–10% Co/Al2O3 (see Fig. 8b) as it was most likely overlapped by the broad carbon peak.
Raman spectra of spent 10% Co/Al2O3 and 3% Ce–10% Co/Al2O3 in Fig. 9 show two distinctive peaks at around 1340.8 and 1580.1 cm−1. These peaks were attributed to D-band (1340.8 cm−1) representing the disordered structural form of amorphous or filamentous carbons, and G band (1580.1 cm−1) conforming to ordered graphite [28, 29]. The extent of carbon crystallinity can be justified using the relative integrated area of D–G bands (ID/IG). A small ID/IG value demonstrates high crystallinity because of dominant amounts of graphitized carbon [30]. The 10% Co/Al2O3 showed a lower graphitization extent (ID/IG = 2.20) than 3% Ce–10% Co/Al2O3 (ID/IG = 1.63). However, as seen in Fig. 9, the peak intensity of unpromoted catalyst was superior to Ce-doped catalyst for both D- and G bands. The low Raman signal intensity for 3% Ce–10% Co/Al2O3 proved the catalyst tolerance towards coking compared to 10% Co/Al2O3. This observation could be ascribed to the CeO2 redox properties resulting in simultaneous reduction of carbonaceous species from catalyst surface during EGDR [25].
Figure 10 displays the TPO profiles with respect to temperature for spent 10% Co/Al2O3 and 3% Ce–10% Co/Al2O3 after EGDR at stoichiometric feed ratio (\(F_{{{\text{CO}}_{ 2} }} = F_{{{\text{C}}_{ 2} {\text{H}}_{ 6} {\text{O}}_{ 2} }}\)) and T = 998 K. For both catalysts, the obvious weight drop was detected within the temperature range of 748–823 K. The spent 10% Co/Al2O3 possessed a higher carbon content of 96.6% than that of 3% Ce–10% Co/Al2O3 (89.9%). The reduction in carbonaceous deposits was induced by the high basic property and great oxygen mobility of Ce promoter [11, 25]. Siang et al. also proposed mechanistic steps [see Eqs. (15) and (16)] for the removal of carbonaceous deposits (CxHy) from catalyst surface based on the redox cycling of CeO2 dopant [31]. The drop in carbon deposits on 3% Ce–10% Co/Al2O3 could clarify its superior catalytic stability with time on stream in comparison with 10% Co/Al2O3 (see Fig. 7):
Conclusions
It is the first time that EGDR reaction was successfully conducted to yield syngas using 10% Co/Al2O3 and 3% Ce–10% Co/Al2O3 catalysts. The addition of Ce promoter reduced the crystallite size of Co3O4, facilitated Co3O4 reduction in H2 and improved the catalytic performance of 10% Co/Al2O3. Increasing reaction temperature from 923 to 998 K enhanced catalytic activity and achieved the highest EG conversion of 71.7% and H2 yield of 69.3% at 998 K on 3% Ce–10% Co/Al2O3. The H2/CO ratio varied from 0.98 to 1.25 suitable for high-molecular weight hydrocarbon synthesis via FTS in downstream processes. The yield of CH4 intermediate product was trivial since it was subsequently converted into final H2 and CO gases by methane dry and steam reforming reactions.
References
Ni M, Leung DYC, Leung MKH (2007) A review on reforming bio-ethanol for hydrogen production. Int J Hydrog Energy 32:3238–3247
Li D, Li X, Gong J (2016) Catalytic reforming of oxygenates: state of the art and future prospects. Chem Rev 116:11529–11653
Chheda JN, Huber GW, Dumesic JA (2007) Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew Chem Int Ed 46:7164–7183
Abdullah B, Ghani NAA, Vo D-VN (2017) Recent advances in dry reforming of methane over Ni-based catalysts. J Clean Prod 162:170–185
Vo D-VN, Adesina AA (2011) Kinetics of the carbothermal synthesis of Mo carbide catalyst supported on various semiconductor oxides. Fuel Process Technol 92:1249–1260
Cabulong RB, Valdehuesa KNG, Ramos KRM, Nisola GM, Lee W-K, Lee CR, Chung W-J (2017) Enhanced yield of ethylene glycol production from d-xylose by pathway optimization in Escherichia coli. Enzyme Microb Technol 97:11–20
Pereira B, Zhang H, De Mey M, Lim CG, Li Z-J, Stephanopoulos G (2016) Engineering a novel biosynthetic pathway in Escherichia coli for production of renewable ethylene glycol. Biotechnol Bioeng 113:376–383
Wang S, Chen J, Chen L (2014) Selective conversion of cellulose into ethylene glycol over metal-organic framework-derived multifunctional catalysts. Catal Lett 144:1728–1734
Koichumanova K, Vikla AK, de Vlieger DJ, Seshan K, Mojet BL, Lefferts L (2013) Towards stable catalysts for aqueous phase conversion of ethylene glycol for renewable hydrogen. Chemsuschem 6:1717–1723
Sohn H, Ozkan US (2016) Cobalt-based catalysts for ethanol steam reforming: an overview. Energy Fuels 30:5309–5322
Fayaz F, Danh HT, Nguyen-Huy C, Vu KB, Abdullah B, Vo D-VN (2016) Promotional effect of Ce-dopant on Al2O3-supported Co catalysts for syngas production via CO2 reforming of ethanol. Procedia Eng 148:646–653
Ayodele BV, Khan MR, Cheng CK (2016) Catalytic performance of ceria-supported cobalt catalyst for CO-rich hydrogen production from dry reforming of methane. Int J Hydrog Energy 41:198–207
Budiman AW, Song S-H, Chang T-S, Shin C-H, Choi M-J (2012) Dry reforming of methane over cobalt catalysts: a literature review of catalyst development. Catal Surv Asia 16:183–197
Mei D, Lebarbier Dagle V, Xing R, Albrecht KO, Dagle RA (2016) Steam reforming of ethylene glycol over MgAl2O4 supported Rh, Ni, and Co catalysts. ACS Catal 6:315–325
Chu X, Liu J, Sun B, Dai R, Pei Y, Qiao M, Fan K (2011) Aqueous-phase reforming of ethylene glycol on Co/ZnO catalysts prepared by the coprecipitation method. J Mol Catal A 335:129–135
Batista MS, Santos RKS, Assaf EM, Assaf JM, Ticianelli EA (2003) Characterization of the activity and stability of supported cobalt catalysts for the steam reforming of ethanol. J Power Sources 124:99–103
Yu S-W, Huang H-H, Tang C-W, Wang C-B (2014) The effect of accessible oxygen over Co3O4–CeO2 catalysts on the steam reforming of ethanol. Int J Hydrog Energy 39:20700–20711
Pardo-Tarifa F, Cabrera S, Sanchez-Dominguez M, Boutonnet M (2017) Ce-promoted Co/Al2O3 catalysts for Fischer–Tropsch synthesis. Int J Hydrog Energy 42:9754–9765
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56:978–982
Fischer N, van Steen E, Claeys M (2011) Preparation of supported nano-sized cobalt oxide and fcc cobalt crystallites. Catal Today 171:174–179
Liu Y, Jia L, Hou B, Sun D, Li D (2017) Cobalt aluminate-modified alumina as a carrier for cobalt in Fischer–Tropsch synthesis. Appl Catal A 530:30–36
Moodley DJ, Saib AM, van de Loosdrecht J, Welker-Nieuwoudt CA, Sigwebela BH, Niemantsverdriet JW (2011) The impact of cobalt aluminate formation on the deactivation of cobalt-based Fischer–Tropsch synthesis catalysts. Catal Today 171:192–200
(2000) JCPDS Powder Diffraction File. International Centre for Diffraction Data, Swarthmore
Braga TP, Essayem N, Valentini A (2017) Correlation between the basicity of Cu–MxOy–Al2O3 (M = Ba, Mg, K or La) oxide and the catalytic performance in the glycerol conversion from adsorption microcalorimetry characterization. J Therm Anal Calorim 129:65–74
Amin R, Chang X, Liu B (2017) Synergistic effect of CeO2 in CH4/CO2 dry reforming reaction over stable xCeO2 yNi/MCM-22 catalysts. Ind Eng Chem Res 56:7445–7453
Kamonsuangkasem K, Therdthianwong S, Therdthianwong A, Thammajak N (2017) Remarkable activity and stability of Ni catalyst supported on CeO2–Al2O3 via CeAlO3 perovskite towards glycerol steam reforming for hydrogen production. Appl Catal B 218:650–663
Siang TJ, Pham TLM, Cuong NV, Phuong PTT, Phuc NHH, Truong QD, Vo D-VN (2018) Combined steam and CO2 reforming of methane for syngas production over carbon-resistant boron-promoted Ni/SBA-15 catalysts. Microporous Mesoporous Mater 262:122–132
Liu W-W, Chai S-P, Mohamed AR, Hashim U (2014) Synthesis and characterization of graphene and carbon nanotubes: a review on the past and recent developments. J Indus Eng Chem 20:1171–1185
Bahari MB, Phuc NHH, Alenazey F, Vu KB, Ainirazali N, Vo D-VN (2017) Catalytic performance of La-Ni/Al2O3 catalyst for CO2 reforming of ethanol. Catal Today 291:67–75
Paksoy AI, Caglayan BS, Ozensoy E, Ökte AN, Aksoylu AE (2018) The effects of Co/Ce loading ratio and reaction conditions on CDRM performance of CoCe/ZrO2 catalysts. Int J Hydrog Energy 43:4321–4334
Siang TJ, Danh HT, Singh S, Truong QD, Setiabudi HD, Vo DVN (2017) Syngas production from combined steam and carbon dioxide reforming of methane over Ce-modified silica-supported nickel catalysts. Chem Eng Trans 56:1129–1134
Acknowledgements
The research fund (UMP Research Grant Scheme, RDU170326) from Universiti Malaysia Pahang to carry out this work is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jun, L.N., Bahari, M.B., Phuong, P.T.T. et al. Ethylene glycol dry reforming for syngas generation on Ce-promoted Co/Al2O3 catalysts. Appl Petrochem Res 8, 253–261 (2018). https://doi.org/10.1007/s13203-018-0223-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13203-018-0223-8