Abstract
Objective
To compare the diagnostic performance of abbreviated MRI (AMRI) combined with multiphasic CT (mCT) with that of full-sequence gadoxetic acid-enhanced MRI (EOB-MRI) in a hepatocellular carcinoma (HCC)-screening cohort
Methods
Consecutive patients at risk of HCC who underwent EOB-MRI and mCT within 3 months for evaluation of new 0.5–3-cm hepatic observations were retrospectively recruited from 3 centers. An AMRI protocol comprising hepatobiliary phase, T2- and diffusion-weighted imaging, and dual-echo sequence was reconstituted from EOB-MRI. Two radiologists independently reviewed each observation in AMRI plus mCT (set 1) and EOB-MRI (set 2) per LI-RADS v2018. Per-lesion sensitivity, accuracy, and positive predictive value (PPV) for HCC were calculated and compared between image sets.
Results
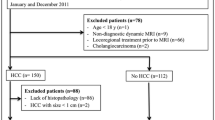
In 267 patients, 306 histologically confirmed observations (280 HCCs, 20 combined hepatocellular-cholangiocarcinomas, 1 cholangiocarcinoma, and 5 benignities) were assessed. Set 1 yielded higher sensitivity (96.4% vs. 92.9%, p = 0.013) and comparable accuracy (91.2% vs. 87.6%) and PPV (94.1% vs. 93.5%) to set 2 using LI-RADS category (LR)-4/5 criteria. The sets showed comparable sensitivity (66.4% vs. 70.4%), accuracy (67.7% vs. 70.6%), and PPV (97.4% vs. 96.6%) using LR-5 criteria. A similar substantial number of non-HCC malignancies were categorized as LR-4 or LR-5, as was the number of HCCs categorized as LR-M in both sets.
Conclusions
AMRI combined with mCT showed diagnostic performance similar or superior to that of EOB-MRI for HCC diagnosis using LI-RADS. Therefore, mCT holds potential as a sequential examination for HCC diagnosis in AMRI-detected hepatic observation in patients at risk of HCC.
Key Points
• AMRI plus multiphasic CT showed comparable accuracy (91.2%) and PPV (94.1%) to full-sequence gadoxetic acid-enhanced MRI using LR-4/5 criteria.
• AMRI plus multiphasic CT was significantly more sensitive than full-sequence gadoxetic acid-enhanced MRI (96.4% vs. 92.9%) using LR-4/5 criteria.
• Multiphasic CT is a potential sequential modality for HCC diagnosis after AMRI.



Similar content being viewed by others
Abbreviations
- AMRI:
-
Abbreviated magnetic resonance imaging
- CC:
-
Cholangiocarcinoma
- cHCC-CC:
-
Combined hepatocellular-cholangiocarcinoma
- DN:
-
Dysplastic nodule
- Dual-GRE:
-
T1-weighted in-phase and out-of-phase images
- DWI:
-
Diffusion-weighted images
- EOB-MRI:
-
Full-sequence gadoxetic acid-enhanced MRI
- FN:
-
False negative
- FP:
-
False positive
- HBP:
-
T1-weighted hepatobiliary phase
- HCC:
-
Hepatocellular carcinoma
- LI-RADS:
-
Liver Imaging Reporting and Data System
- LR:
-
LI-RADS category
- mCT:
-
Multiphasic computed tomography
- MRI:
-
Magnetic resonance imaging
- PPV:
-
Positive predictive value
- T2WI:
-
T2-weighted images
- TN:
-
True negative
- TP:
-
True positive
References
European Association for the Study of the Liver (2018) EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 69:182–236
Fattovich G, Stroffolini T, Zagni I, Donato F (2004) Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 127:S35–S50
Omata M, Cheng AL, Kokudo N et al (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11:317–370
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750
Colli A, Fraquelli M, Casazza G et al (2006) Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol 101:513–523
Singal A, Volk ML, Waljee A et al (2009) Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 30:37–47
Besa C, Lewis S, Pandharipande PV et al (2017) Hepatocellular carcinoma detection: diagnostic performance of a simulated abbreviated MRI protocol combining diffusion-weighted and T1-weighted imaging at the delayed phase post gadoxetic acid. Abdom Radiol (NY) 42:179–190
Han S, Choi JI, Park MY, Choi MH, Rha SE, Lee YJ (2018) The diagnostic performance of liver MRI without intravenous contrast for detecting hepatocellular carcinoma: a case-controlled feasibility study. Korean J Radiol 19:568–577
Kim YK, Kim YK, Park HJ, Park MJ, Lee WJ, Choi D (2014) Noncontrast MRI with diffusion-weighted imaging as the sole imaging modality for detecting liver malignancy in patients with high risk for hepatocellular carcinoma. Magn Reson Imaging 32:610–618
Marks RM, Ryan A, Heba ER et al (2015) Diagnostic per-patient accuracy of an abbreviated hepatobiliary phase gadoxetic acid-enhanced MRI for hepatocellular carcinoma surveillance. AJR Am J Roentgenol 204:527–535
Tillman BG, Gorman JD, Hru JM et al (2017) Diagnostic per-lesion performance of a simulated gadoxetate disodium-enhanced abbreviated MRI protocol for hepatocellular carcinoma screening. Clin Radiol
Fidler J, Hough D (2011) Hepatocyte-specific magnetic resonance imaging contrast agents. Hepatology 53:678–682
Davenport MS, Caoili EM, Kaza RK, Hussain HK (2014) Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology 272:123–131
Chernyak V, Fowler KJ, Kamaya A et al (2018) Liver imaging reporting and data system (LI-RADS) version 2018: imaging of hepatocellular carcinoma in at-risk patients. Radiology:181494
Sirlin C (2017) Use of the liver imaging reporting and data system in hepatocellular carcinoma. Gastroenterol Hepatol (N Y) 13:363–365
Basha MAA, AlAzzazy MZ, Ahmed AF et al (2018) Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? A prospective comparative study. Eur Radiol 28:2592–2603
Cha DI, Jang KM, Kim SH, Kang TW, Song KD (2017) Liver imaging reporting and data system on CT and gadoxetic acid-enhanced MRI with diffusion-weighted imaging. Eur Radiol 27:4394–4405
Shin Y, Kim KW, Lee AJ et al (2019) A good practice–compliant clinical trial imaging management system for multicenter clinical trials: development and validation study. JMIR Med Inform 7:e14310
CT/MRI LIRADS® v2018 CORE - American College of Radiology. Available at https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.pdf?la=en Last accessed June 20 2019
Kundel HL, Polansky M (2003) Measurement of observer agreement. Radiology 228:303–308
Feinstein AR, Cicchetti DV (1990) High agreement but low kappa: I. The problems of two paradoxes. J Clin Epidemiol 43:543–549
Kim YY, An C, Kim S, Kim MJ (2018) Diagnostic accuracy of prospective application of the Liver Imaging Reporting and Data System (LI-RADS) in gadoxetate-enhanced MRI. Eur Radiol 28:2038–2046
Yoon JH, Lee JM, Lee YJ, Lee KB, Han JK (2019) Added value of sequentially performed gadoxetic acid-enhanced liver MRI for the diagnosis of small (10-19 mm) or atypical hepatic observations at contrast-enhanced CT: a prospective comparison. J Magn Reson Imaging 49:574–587
Zhang YD, Zhu FP, Xu X et al (2016) Classifying CT/MR findings in patients with suspicion of hepatocellular carcinoma: comparison of liver imaging reporting and data system and criteria-free Likert scale reporting models. J Magn Reson Imaging 43:373–383
Kim YY, Kim MJ, Kim EH, Roh YH, An C (2019) Hepatocellular carcinoma versus other hepatic malignancy in cirrhosis: performance of LI-RADS Version 2018. Radiology 291:72–80
Park SH, Lee SS, Yu E et al (2017) Combined hepatocellular-cholangiocarcinoma: gadoxetic acid-enhanced MRI findings correlated with pathologic features and prognosis. J Magn Reson Imaging 46:267–280
Choi SH, Lee SS, Park SH et al (2018) LI-RADS classification and prognosis of primary liver cancers at gadoxetic acid–enhanced MRI. Radiology 290:388–397
Jeon SK, Joo I, Lee DH et al (2019) Combined hepatocellular cholangiocarcinoma: LI-RADS v2017 categorisation for differential diagnosis and prognostication on gadoxetic acid-enhanced MR imaging. Eur Radiol 29:373–382
Lima PH, Fan B, Bérubé J et al (2019) Cost-utility analysis of imaging for surveillance and diagnosis of hepatocellular carcinoma. AJR Am J Roentgenol 213:17–25
Andersson KL, Salomon JA, Goldie SJ, Chung RT (2008) Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 6:1418–1424
Lee JY, Huo EJ, Weinstein S et al (2018) Evaluation of an abbreviated screening MRI protocol for patients at risk for hepatocellular carcinoma. Abdom Radiol (NY) 43:1627–1633
Funding
This study was supported by a research fund from the Korean Society of Radiology through Radiology Imaging Network of Korea for Clinical Research (RINK-CR) and the Scientific Research Fund of the Korean Liver Cancer Study Group.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Bohyun Kim.
Conflict of interest
The authors declare that they have no conflicts of interest.
Statistics and biometry
Bohyun Kim, MD, PhD, who is one of the authors, has significant statistical expertise.
Informed consent
The requirement for written informed consent was waived for this study because this retrospective study was approved by the institutional review board of three institutions.
Ethical approval
Institutional review board approval of Asan Medical Center, Gil Medical Center, and Ajou University Hospital was obtained.
Methodology
• retrospective
• diagnostic or prognostic study
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 25 kb)
Rights and permissions
About this article
Cite this article
Park, S.H., Kim, B., Kim, S.Y. et al. Abbreviated MRI with optional multiphasic CT as an alternative to full-sequence MRI: LI-RADS validation in a HCC-screening cohort. Eur Radiol 30, 2302–2311 (2020). https://doi.org/10.1007/s00330-019-06546-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06546-5