Abstract
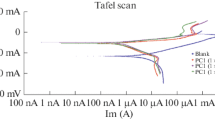
Cyclic voltammetry and potentiodynamic polarization techniques were used to study the effects of 4-[4-(1,3-benzothiazol-2yl)phenoxy] phthalonitrile (BT) and tetrakis[(benzo[d]thiazol-2ylphenoxy) phthalocyaninato] gallium(III)chloride (ClGaBTPc) as aluminium corrosion inhibitors in 1.0 M hydrochloric acid. The presence of the inhibitors in the concentration range of 2 to 10 μM was found to retard the aluminium corrosion process such that the inhibition efficiency was found to range from 28.2 to 76.1% for BT and from 71.5 to 82.7% for ClGaBTPc. The latter was a better inhibitor. Scanning electron microscopy and energy-dispersive X-ray measurements reveal effective metal surface protection by the inhibitors, most probably by shielding it from the corrosion attacks of Cl− from the acid. The calculated quantum chemical parameters agreed with experimental results.

Adsorption of benzothiazole phthalocyanine onto the metal surface for the protection of the metal from the aggressive attack of Cl−.












Similar content being viewed by others
Data Availability
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical or time limitations.
References
P. Deepa, R. Padmalatha, Corrosion behaviour of 6063 aluminium alloy in acidic and in alkaline media. Arab. J. Chem. 10, S2234–S2244 (2017)
A.K. Mishra, R. Balasubramanian, Corrosion inhibition of aluminum alloy AA 2014 by rare earth chlorides. Corros. Sci. 49(3), 1027–1044 (2007)
S.G. Aziz, A.A. El-Awady, B.A. Abd-El-Nabey, Studies on the kinetics of aluminium metal dissolution in aggressive media containing inorganic anions. J. King Abdulaziz Univ. (SAU) Sci. 9(1), 101–115 (1997)
L.T. Popoola, A.S. Grema, G.K. Latinwo, B. Gutti, A.S. Balogun, Corrosion problems during oil and gas production and its mitigation. Int. J. Ind. Chem. 4(35), 35 (2013). https://doi.org/10.1186/2228-5547-4-35
J.W.J. Silva, E.N. Codaro, R.Z. Nakazato, L.R.O. Hein, Influence of chromate, molybdate and tungstate on pit formation in chloride medium. Appl. Surf. Sci. 252(4), 1117–1122 (2005)
K. Xhanari, M. Finšgar, M.K. Hrnčič, U. Maver, Z. Knez, B. Seiti, Green corrosion inhibitors for aluminium and its alloys: a review. RSC Adv. 7(44), 27299–27330 (2017)
S.A.M. Refaey, S.S.A. El-Rehim, F. Taha, M.B. Saleh, R.A. Ahmed, Inhibition of chloride localized corrosion of mild steel by \( P{O}_4^{3-} \), \( Cr{O}_4^{2-} \), \( Mo{O}_4^{2-} \) and \( N{O}_2^{-} \) anions. Appl. Surf. Sci. 158(3–4), 190–196 (2000)
A. Aballe, M. Bethencourt, F.J. Botana, M. Marcos, CeCl and LaCl binary solutions as environment-friendly corrosion inhibitors of AA5083 Al–Mg alloy in NaCl solutions. J. Alloys Compd. 323-324, 855–858 (2001)
D. Eaves, G. Williams, H.N. McMurray, Inhibition of self-corrosion in magnesium by poisoning hydrogen recombination on iron impurities. Electrochim. Acta 79, 1–7 (2012)
M. Iannuzzi, G.S. Frankel, Mechanisms of corrosion inhibition of AA2024-T3 by vanadates. Corros. Sci. 49(5), 2371–2391 (2007)
N.J.N. Nnaji, O.T. Ujam, N.E. Ibisi, J.U. Ani, T.O. Onuegbu, L.O. Olasunkanmi, E.E. Ebenso, Morpholine and piperazine based carboxamide derivatives as corrosion inhibitors of mild steel in HCl medium. J. Mol. Liq. 230, 652–661 (2017)
I.B. Obot, N.O. Obi-Egbedi, S.A. Umoren, Adsorption characteristics and corrosion inhibitive properties of clotrimazole for aluminium corrosion in hydrochloric acid. Int. J. Electrochem. Sci. 4, 863–877 (2009)
S.A. Abd El-Maksoud, The effect of organic compounds on the electrochemical behaviour of steel in acidic media. A review. Int. J. Electrochem. Sci. 3, 528–555 (2008)
S.A. Umoren, U.M. Eduok, Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: A review. Carbohydr. Polym. 140, 314–341 (2016)
N.J. Nnaji, N.O. Obi-Egbedi, M.A. Nnabugwu, Kinetics and thermodynamics of aluminium corrosion by solution in the presence of Anthocleista djalonensis leaf extract. Int. J. Chem. Sci. 10(1), 182–194 (2012)
M. Dibetsoe, L.O. Olasunkanmi, O.E. Fayemi, S. Yesudass, B. Ramaganthan, I. Bahadur, A.S. Adekunle, M.M. Kabanda, E.E. Ebenso, Some phthalocyanine and naphthalocyanine derivatives as corrosion inhibitors for aluminium in acidic medium: experimental, quantum chemical calculations, qsar studies and synergistic effect of iodide ions. Molecules 20(9), 15701–15734 (2015)
O.K. Özdemir, A. Aytaç, D. Atilla, M. Durmuş, Corrosion inhibition of aluminum by novel phthalocyanines in hydrochloric acid solution. J. Mater. Sci. 46(3), 752–758 (2011)
A. Aktaş, M. Durmuş, L. Değrmencioğlu, Self-assembling novel phthalocyanines containing a rigid benzothiazole skeleton with a 1,4-benzene linker: synthesis, spectroscopic and spectral properties, and photochemical/photophysical affinity. Polyhedron 48(1), 80–91 (2012)
N. Nwaji, O.M. Bankole, J. Britton, T. Nyokong, Photophysical and nonlinear optical study of benzothiazole substituted phthalocyanines in solution and thin films. J. Porphyrins Phthalocyanines 21(4–6), 263–272 (2017)
S.S.A. El-Rehim, S.A.M. Refaey, F. Taha, M.B. Saleh, R.A. Ahmed, Corrosion inhibition of mild steel in acidic medium using 2-amino thiophenol and 2-cyanomethyl benzothiazole. J. Appl. Electrochem. 31(4), 429–435 (2001)
M. Yadav, S. Kumar, N. Kumari, I. Bahadur, E.E. Ebenso, Experimental and theoretical studies on corrosion inhibition effect of synthesized benzothiazole derivatives on mild steel in 15% HCl solution. Int. J. Electrochem. Sci. 10, 602–624 (2015)
A.S. Fouda, M. Diab, A. El-Sonbati, S.A. Hassan, Benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M phosphoric acid (H3PO4) solutions. Afr. J. Pure Appl. Chem. 7(2), 67–78 (2013)
Z. Salarvand, M. Amirnasr, M. Talebian, K. Raeissi, S. Meghdadia, Enhanced corrosion resistance of mild steel in 1 M HCl solution by trace amount of 2-phenyl-benzothiazole derivatives: experimental, quantum chemical calculations and molecular dynamics (MD) simulation studies. Corros. Sci. 114, 133–145 (2017)
Z. Hu, Y. Meng, X. Ma, H. Zhu, J. Li, C. Li, D. Cao, Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HCl. Corros. Sci. 112, 563–575 (2016)
I.V. Aoki, I.G. Guedes, S.L. Maranhao, Copper phthalocyanine as corrosion inhibitor for ASTM A606-4 steel in 16% hydrochloric acid. J. Appl. Electrochem. 32(8), 915–919 (2002)
P. Zhao, Q. Liang, Y. Li, Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/L HCl. Appl. Surf. Sci. 252(5), 1596–1607 (2005)
J.P. Chen, X.S. Zhang, Phosphorous phthalocyanine analogues as degradable corrosion inhibitor of mild steel in 1 mol/L HCl. Trans. Indian Inst. Metals 71(5), 1113–1126 (2018)
E. Sezer, B. Ustamehmetoglu, Z.A. Bayır, K. Coban, A. Kalkan, Corrosion inhibition effect of 4-(2-diethylamino-ethylsulfonyl)-phthalonitrile and 4,5-bis(hexylsulfonyl)-phthalonitrile. Int. J. Electrochem 2011, Article ID 235360, 5 pages (2011)
S. Ghareba, S. Omanovic, The effect of electrolyte flow on the performance of 12-aminododecanoic acid as a carbon steel corrosion inhibitor in CO2-saturated hydrochloric acid. Corros. Sci. 53(11), 3805–3812 (2011)
S. Ghareba, S. Omanovic, 12-Aminododecanoic acid as a corrosion inhibitor for carbon steel. Electrochim. Acta 56(11), 3890–3898 (2011)
Z. Shirazi, M.H. Keshavarz, K. Esmaeilpour, T. Pakniya, A novel and simple method for the prediction of corrosion inhibition efficiency without using complex computer codes. Z. Anorg. Allg. Chem. 643(24), 2149–2157 (2017)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, G. Cheeseman, J.R. Scalmani, V. Barone, B. Mennucci, H. Petersson, G.A. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, K. Hada, M. Ehara, M. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.E. Montgomery, J.A. Peralta Jr., F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, R. Staroverov, V.N. Kobayashi, K. Normand, J. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, S. Dannenberg, J.J. Dapprich, A.D. Daniels, Ö. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian 09, Revision E. 01, Gaussian Inc, Wallingford CT, 2009.
B. Xu, W. Yang, Y. Liu, X. Yin, W. Gong, Y. Chen, Experimental and theoretical evaluation of two pyridinecarboxaldehyde thiosemicarbazone compounds as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 78, 260–268 (2014)
Y. Yan, W. Li, L. Cai, B. Hau, Electrochemical and quantum chemical study of purines as corrosion inhibitors for mild steel in 1 M HCl solution. Electrochim. Acta 53(20), 5953–5960 (2008)
M.E. Mashuga, L.O. Olasunkanmi, A.S. Adekunle, S. Yesudass, M.M. Kabanda, E.E. Ebenso, Adsorption, thermodynamics and quantum chemical studies of 1-hexyl-3-methylimidazolium based ionic liquids as corrosion inhibitors for mild steel in HCl. Materials 8(6), 3607–3632 (2015)
M. Abdallah, B.H. Asghar, I. Zaafarany, A.S. Fouda, The inhibition of carbon steel corrosion in hydrochloric acid solution using some phenolic compounds. Int. J. Electrochem. Sci. 7, 282–304 (2012)
Z. Pilic, I. Martinovic, Y. Yan, W. Li, L. Cai, B. Hau, A comparative study on the electrochemical behaviour of aluminium and 8090 Al- Li-Cu-Mg alloy in acid rain solution. Int. J. Electrochem. Sci. 12, 3576–3588 (2017)
N.J.N. Nnaji, C.O.B. Okoye, N.O. Obi-Egbedi, M.A. Ezeokonkwo, J.U. Ani, Spectroscopic characterization of red onion skin tannin and it’s use as alternative aluminium corrosion inhibitor in hydrochloric acid solutions. Int. J. Electrochem. Sci. 8, 1735–1758 (2013)
N.J.N. Nnaji, N.O. Obi-Egbedi, C.O.B. Okoye, Cashew nut testa tannin: assessing its effects on the corrosion of aluminium in HCl. Port. Electrochim. Acta 32(2), 157–182 (2014)
K. Leetmaa, M.A. Gomez, L. Becze, F. Guo, G.P. Demopoulos, Comparative molecular characterization of aluminum hydroxy-gels derived from chloride and sulphate salts. J. Chem. Technol. Biotechnol. 89(2), 206–213 (2014)
A. Handy, N.S. El-Gendy, Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egypt. J. Pet. 22(1), 17–25 (2013)
K.A. Rodgers, M.R. Gregory, R. Barton, Bayerite, Nordstrandite, Gibbsite, Brucite, and Pseudoboehmite in discharged caustic waste from Campbell Island, Southwest Pacific. Clay. Clay Miner. 39(1), 103–107 (1991)
T. Kozawa, M. Naito, Mechanically induced formation of metastable χ- and κ-Al2O3 from boehmite. Adv. Powder Technol. 27(3), 935–939 (2016)
A.W. Snow, J.R. Griffith, N.P. Marullo, Syntheses and characterization of heteroatom-bridged metal free phthalocyanine network polymers and model compounds. Macromolecules 17(8), 1614–1624 (1984)
K. Görgün, H.C. Sakarya, M. Özkütük, The synthesis, characterization, acid dissociation, and theoretical calculation of several novel benzothiazole schiff base derivatives. J. Chem. Eng. Data 60(3), 594–601 (2015)
R.T. Wheelhouse, D.F. Shi, D.E.V. Wilman, M.F.G. Stevens, Antitumour benzothiazoles. Part 4. An NMR study of the sites of protonation of 2-(4-aminophenyl)benzothiazoles. J. Chem. Soc., Perkin Trans. 2(7), 1271–1274 (1996)
Y.H. So, J.M. Zaleski, C. Murlick, A. Ellaboudy, Synthesis and photophysical properties of some benzoxazole and benzothiazole compounds. Macromolecules 29(8), 2783–2795 (1996)
P.A. Bernstein, A.B.P. Lever, Protonation of cobalt tetraneopentoxyphthalocyanine as a function of oxidation state. Inorg. Chim. Acta 198-200, 543–555 (1992)
I.M. Lipatova, A.A. Yusova, E.A. Lukyanets, Supramolecular complexation of the cationic derivative of Zn (II) phthalocyanine and sodium alginate in mixed aqueous solutions. J. Photochem. Photobiol. A Chem. 364, 588–594 (2018)
H. Gerengi, M. Mielniczek, G. Gece, M.M. Solomon, Experimental and quantum chemical evaluation of 8-hydroxyquinoline as a corrosion inhibitor for copper in 0.1 M HCl. Ind. Eng. Chem. Res. 55(36), 9614–9624 (2016)
J. Haque, C. Verma, V. Srivastava, M.A. Quraishi, E.E. Ebenso, Experimental and quantum chemical studies of functionalized tetrahydropyridines as corrosion inhibitors for mild steel in 1M hydrochloric acid. Results Phys. 9, 1481–1493 (2018)
J.O. Bockris, D.A.J. Swinkels, Adsorption of naphthalene on solid metal electrodes. J. Electrochem. Soc. 111(6), 743–748 (1964)
N.O. Obi-Egbedi, I.B. Obot, Xanthione: a new and effective corrosion inhibitor for mild stell in sulphuric acid solution. Arab. J. Chem. 6(2), 211–223 (2013)
E.E. Oguzie, Y. Li, F.H. Wang, Corrosion inhibition and adsorption behaviour of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid Interface Sci. 310(1), 90–98 (2007)
L.O. Olasunkanmi, I.B. Obot, E.E. Ebenso, Adsorption and corrosion inhibition properties of N-{n-[1-R-5-(quinoxalin-6-yl)-4,5-dihydropyrazol-3-yl]phenyl}methanesulfonamides on mild steel in 1 M HCl: experimental and theoretical studies. RSC Adv. 6(90), 86782–86797 (2016)
M. Prajila, P.R. Ammal, A. Joseph, Comparative studies on the corrosion inhibition characteristics of three different triazine based Schiff’s bases, HMMT, DHMMT and MHMMT, for mild steel exposed in sulfuric acid. Egypt. J. Pet. 27(4), 467–475 (2017)
I.B. Obot, N.O. Obi-Egbedi, S.A. Umoren, The synergistic inhibitive effect and some quantum chemical parameters of 2,3-diaminonaphthalene and iodide ions on the hydrochloric acid corrosion of aluminium. Corros. Sci. 51(2), 276–282 (2009)
S. Shen, C.D. Zhu, X.Y. Guo, C.C. Li, Y. Wen, H.F. Yang, The synergistic mechanism of phytic acid monolayers and iodide ions for inhibition of copper corrosion in acidic media. RSC Adv. 4(21), 10597–10606 (2014)
T.I. Strelkova, G.P. Gurinovich, G.N. Sinyakov, Investigation of the ionization of phthalocyanines by luminescence spectra. J. Appl Spectros. 4(5), 313-315 (1996)
D.L. Ledson, M.V. Twigg, Acid-base behaviour of phthalocyanine. Inorg. Chim. Acta 13, 43–46 (1975)
A.Y. El-Etre, Inhibition of aluminum corrosion using Opuntia extract. Corros. Sci. 45(11), 2485–2495 (2003)
E.E. Oguzie, Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract. Corros. Sci. 49(3), 1527–1539 (2007)
P.C. Okafor, M.E. Ikpi, I.E. Uwah, E.E. Ebenso, U.J. Ekpe, S.A. Umoren, Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 50(8), 2310–2317 (2008)
Acknowledgements
This work was supported by the Department of Science and Technology (DST) and National Research Foundation (NRF), South Africa, through the DST/NRF South African Research Chairs Initiative for Professor of Medicinal Chemistry and Nanotechnology (UID 62620) as well as the Rhodes University/DST Centre for Nanotechnology Innovation, South Africa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 2246 kb)
Rights and permissions
About this article
Cite this article
Nnaji, N., Nwaji, N., Fomo, G. et al. Inhibition of Aluminium Corrosion Using Benzothiazole and Its Phthalocyanine Derivative. Electrocatalysis 10, 445–458 (2019). https://doi.org/10.1007/s12678-019-00538-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12678-019-00538-1