Abstract
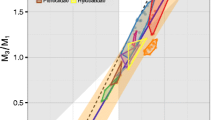
The inhibitory cascade (IC) model is a widely used evolutionary developmental explanation of among-species differences in relative molar tooth size. The IC model posits that, as molars develop from front to back, the relative strength of activating and inhibiting influences establishes a “ratcheting” mechanism leading to predictable relative molar sizes. Such a constraint on molar covariation would lead to strong variational biases on the evolutionary paths that the molar row can traverse through phenotypic space. These constraints manifest themselves in characteristic patterns of variation among species that loosely match observed macroevolutionary patterns. In this paper, we write out the predictions of the IC model for within-species covariation in molar size in a framework that unifies evolutionary developmental biological and quantitative genetic perspectives on the evolution of complex traits. We then evaluate these predictions about aspects of molar covariation in eight anthropoid primate species. We find that the IC model tends to over-predict aspects of within-species covariation by substantial margins. Only macaques exhibit covariation in and among individual teeth consistent with the IC model, but they do not show signs of the strong evolutionary constraint predicted by the model. Gorillas meet none of the predictions. While we cannot rule out an IC-like process as a contributor, causes of molar size covariation other than those described in the IC model must be major contributors to covariation in molar teeth within populations.



Similar content being viewed by others
References
Asahara, M. (2013). Unique inhibitory cascade pattern of molars in canids contributing to their potential to evolutionary plasticity of diet. Ecology and Evolution, 3(2), 278–285.
Bernal, V., Gonzalez, P. N., & Perez, S. I. (2013). Developmental processes, evolvability, and dental diversification of New World monkeys. Evolutionary Biology, 40(4), 532–541.
Bohrnstedt, G. W., & Goldberger, A. S. (1969). On the exact covariance of products of random variables. Journal of the American Statistical Association, 64(328), 1439–1442.
Bookstein, F. L. (2016). The inappropriate symmetries of multivariate statistical analysis in geometric morphometrics. Evolutionary Biology, 43, 277–313.
Carter, K. E., & Worthington, S. (2016). The evolution of anthropoid molar proportions. BMC Evolutionary Biology, 16, 116.
Cheverud, J. M. (1984). Quantitative genetics and developmental constraints on evolution by selection. Journal of Theoretical Biology, 110(2), 155–171.
Cheverud, J. M. (1996). Developmental integration and the evolution of pleiotropy. American Zoologist, 36(1), 44–50.
Delezene, L. K. (2015). Modularity of the anthropoid dentition: Implications for the evolution of the hominin canine honing complex. Journal of Human Evolution, 86, 1–12.
Evans, A. R., Daly, E. S., Catlett, K. K., Paul, K. S., King, S. J., Skinner, M. M., et al. (2016). A simple rule governs the evolution and development of hominin tooth size. Nature, 530(7591), 477–480. https://doi.org/10.1038/nature16972.
Fisher, R. A. (1930). The genetical theory of natural selection. Oxford: Oxford University Press.
Galbany, J., Estebaranz, F., Martínez, L. M., Romero, A., De Juan, J., Turbón, D., et al. (2006). Comparative analysis of dental enamel polyvinylsiloxane impression and polyurethane casting methods for sem research. Microscopy Research and Technique, 69(4), 246–252.
Gomez-Robles, A. (2016). Palaeoanthropology: What teeth tell us. Nature, 530(7591), 425–426. https://doi.org/10.1038/530425a.
Goodall, R. H., Darras, L. P., & Purnell, M. A. (2015). Accuracy and precision of silicon based impression media for quantitative areal texture analysis. Scientific Reports, 5, 10800.
Grabowski, M., & Porto, A. (2016). How many more? Sample size determination in studies of morphological integration and evolvability. Methods in Ecology and Evolution, 8, 592–603.
Green, R. M., Fish, J. L., Young, N. M., Smith, F. J., Roberts, B., Dolan, K., et al. (2017). Developmental nonlinearity drives phenotypic robustness. Nature Communications, 8(1), 1970.
Hadfield, J. D., et al. (2010). MCMC methods for multi-response generalized linear mixed models: The MCMCglmm R package. Journal of Statistical Software, 33(2), 1–22.
Hallgrimsson, B., Green, R. M., Katz, D. C., Fish, J. L., Bernier, F. P., Roseman, C. C., et al. (2018). The developmental-genetics of canalization., Seminars in cell & developmental biology Amsterdam: Elsevier.
Hallgrímsson, B., Jamniczky, H., Young, N. M., Rolian, C., Parsons, T. E., Boughner, J. C., et al. (2009). Deciphering the palimpsest: Studying the relationship between morphological integration and phenotypic covariation. Evolutionary Biology, 36(4), 355–376.
Halliday, T. J., & Goswami, A. (2013). Testing the inhibitory cascade model in Mesozoic and Cenozoic mammaliaforms. BMC Evolutionary Biology, 13(1), 79.
Hansen, T., & Houle, D. (2008). Measuring and comparing evolvability and constraint in multivariate characters. Journal of Evolutionary Biology, 21(5), 1201–1219.
Hlusko, L. J., Schmitt, C. A., Monson, T. A., Brasil, M. F., & Mahaney, M. C. (2016). The integration of quantitative genetics, paleontology, and neontology reveals genetic underpinnings of primate dental evolution. Proceedings of the National Academy of Sciences, 113(33), 9262–9267.
Houle, D. (2010). Numbering the hairs on our heads: The shared challenge and promise of phenomics. Proceedings of the National Academy of Sciences, 107(suppl 1), 1793–1799.
Kavanagh, K. D., Evans, A. R., & Jernvall, J. (2007). Predicting evolutionary patterns of mammalian teeth from development. Nature, 449(7161), 427–432.
Labonne, G., Navarro, N., Laffont, R., Chateau-Smith, C., & Montuire, S. (2014). Developmental integration in a functional unit: Deciphering processes from adult dental morphology. Evolution & Development, 16(4), 224–232.
Lewontin, R. C., et al. (1974). The genetic basis of evolutionary change. New York: Columbia University Press.
Lynch, M., Walsh, B., et al. (1998). Genetics and Analysis of Quantitative Traits (Vol. 1). Sunderland, MA: Sinauer.
Mitteroecker, P. (2009). The developmental basis of variational modularity: Insights from quantitative genetics, morphometrics, and developmental biology. Evolutionary Biology, 36(4), 377–385.
Morrissey, M. B. (2015). Evolutionary quantitative genetics of nonlinear developmental systems. Evolution, 69(8), 2050–2066.
Navarro, N., & Maga, A. M. (2018). Genetic mapping of molar size relations identifies inhibitory locus for third molars in mice. Heredity, 121, 1–11.
Plavcan, J. M. (1990). Sexual dimorphism in the dentition of extant anthropoid primates. Ann Arbor: University Microfilms.
Polly, P. D. (2007). Evolutionary biology: Development with a bite. Nature, 449(7161), 413–415.
Polly, P. D. (2008). Developmental dynamics and G-matrices: Can morphometric spaces be used to model phenotypic evolution? Evolutionary Biology, 35(2), 83–96.
Polly, P. D. (2015). Gene networks, occlusal clocks, and functional patches: New understanding of pattern and process in the evolution of the dentition. Odontology, 103(2), 117–125. https://doi.org/10.1007/s10266-015-0208-3.
Rice, S. H. (2002). A general population genetic theory for the evolution of developmental interactions. Proceedings of the National Academy of Sciences, 99(24), 15518–15523.
Schroer, K., & Wood, B. (2015). Modeling the dental development of fossil hominins through the inhibitory cascade. Journal of Anatomy, 226(2), 150–162.
Sokal, R., & Rohlf, F. (1995). Analysis of frequencies. New York: WH Freeman.
Walsh, B., & Blows, M. W. (2009). Abundant genetic variation + strong selection = multivariate genetic constraints: a geometric view of adaptation. Annual Review of Ecology, Evolution, and Systematics, 40, 41–59.
Wolf, J. B. (2002). The geometry of phenotypic evolution in developmental hyperspace. Proceedings of the National Academy of Sciences, 99(25), 15849–15851.
Wolf, J. B. (2003). Genetic architecture and evolutionary constraint when the environment contains genes. Proceedings of the National Academy of Sciences, 100(8), 4655–4660.
Young, N. M. (2013). Macroevolutionary diversity of amniote limb proportions predicted by developmental interactions. Journal of Experimental Zoology Part B, 320(7), 420–427.
Young, N. M., Winslow, B., Takkellapati, S., & Kavanagh, K. (2015). Shared rules of development predict patterns of evolution in vertebrate segmentation. Nature Communications, 6, 6690.
Acknowledgements
This work was partially supported by a National Science Foundation Dissertation Improvement Grant to LKD under Dr. William H. Kimbel (BCS-0852105) and Wenner-Gren Foundation Dissertation Fieldwork Grant (#7884) to LKD. LKD also acknowledges the financial support provided by a Connor Family Faculty Fellowship and from the Office of Research and Development at the University of Arkansas. We thank Drs. Campbell Rolian, Julia Boughner, Peter Ungar, David Strait, Rebecca Green, Benedikt Hallgrímsson, Ben Auerbach, and the Marcucio, Weaver, Steel, and Hallgrímsson lab groups for comments and critical feedback on early versions of this work that greatly improved it. Errors and omissions are our own.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Roseman, C.C., Delezene, L.K. The Inhibitory Cascade Model is Not a Good Predictor of Molar Size Covariation. Evol Biol 46, 229–238 (2019). https://doi.org/10.1007/s11692-019-09480-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-019-09480-y