Abstract
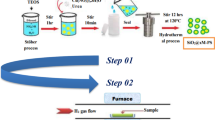
A series of α-Fe2O3 catalysts were prepared by precipitation method by changing calcination temperatures, solvents, precursor concentration, and bases. The structure and morphology of the synthesized catalysts were characterized by SEM, XRD, XPS, and Raman spectroscopy, and the liquid-phase spin conversion of ortho-hydrogen to para-hydrogen was performed by in situ FTIR spectroscopy at cryogenic temperature 17 K. The spin conversion was affected by catalysts calcination temperature, initial precursor concentration, solvents used, and precipitating agent. The highly dispersed and most active α-Fe2O3 was obtained using double-distilled water as solvent and sodium hydroxide solution as precipitating agent followed by calcination at 393 K for 12 h. The formation of hydrated Fe (OH)3 or FeOOH during synthesis of α-Fe2O3 at 393 K dramatically enhanced the catalytic activity and ortho to para-hydrogen spin conversion.










Similar content being viewed by others
References
Hovener JB, Bar S, Leupold J, Jenne K, Leibfritz D, Hennig J, Duckett SB, Von Elverfeldt D (2013) A continuous-flow, high-throughput, high-pressure parahydrogen converter for hyperpolarization in a clinical setting. NMR Biomed 26(2):124–131
Das T, Nah IW, Choi J-G, Oh I-H (2016) Synthesis of iron oxide catalysts using various methods for the spin conversion of hydrogen. Reac Kinet Mech Cat 118:669–681
Kim JH, Karng SW, Oh I-H, Nah IW (2015) Ortho-para hydrogen conversion characteristics of amorphous and mesoporous Cr2O3 powders at a temperature of 77 K. Int J Hydrog Eng 40:14147–14153
Buntkowsky G, Walaszek B, Adamezyk A, Xu Y, Limbach H-H, Chaudret B (2006) Mechanism of nuclear spin initiated para-H2 to ortho-H2 conversion. Phys Chem Chem Phys 8:1929–1935
Balu AM, Duckett SB, Luque R (2009) Para-hydrogen induced polarization effects in liquid phase hydrogenations catalysed by supported metal nanoparticles. Dalton Trans 26:5074–5076
Chekmenev EY, Hovener J, Norton VA, Harris K, Batchelder LS, Bhattacharya P, Ross BD, Weitekamp DP (2008) PASADENA hyperpolarization of succinic acid for MRI and NMR spectroscopy. J Am Chem Soc 130:4212–4213
Van den Berg AWC, Arean CO (2008) Materials for hydrogen storage: current research trends and perspectives. Chem Commun 6:668–681
Pehr K, Sauermann P, Traeger O, Bracha M (2001) Liquid hydrogen for motor vehicles-the world’s first public LH2 filling station. Int J Hydrog Energy 26:777–782
Cheng X-L, Jiang J-S, Jin C-Y, Lin C-C, Zeng Y, Zhang Q-H (2014) Cauliflower-like α-Fe2O3 microstructures: toluene-water interface-assisted synthesis, characterization, and applications in wastewater treatment and visible-light photocatalysis. Chem Eng J 236:139–148
Mohapatra M, Anand S (2010) Synthesis and applications of nano-structured iron oxides/hydroxides -a review. Int J Eng Sci Technol 2(8):127–146
Wang W, Bu F-X, Jiang J-S (2015) Porous TiO2 coated α-Fe2O3 ginger-like nanostructures with enhanced electrochemical properties. Mater Lett 139:89–92
Long NV, Yang Y, Yuasa M, Thi CM, Cao Y, Nann T, Nogami M (2014) Gas-sensing properties of p-type α-Fe2O3 polyhedral particles synthesized via a modified polyol method. RSC Adv 4:8250–8255
Zhu X, Sun L, Zheng Y, Wang H, Wei Y, Li K (2014) CeO2 modified Fe2O3 for the chemical hydrogen storage and production via cyclic water splitting. Int J Hydrog Energy 39:13381–13388
Avila-Garcia A, Carbajal-Franco G (2003) α-Fe2O3 films grown by the spin-on sol-gel deposition method. Rev Mex Fis 49(3):219–223
Das T, Kweon S-C, Choi J-G, Kim SY, Oh I-H (2015) Spin conversion of hydrogen over LaFeO3/Al2O3 catalysts at low temperature: synthesis, characterization and activity. Int J Hydrog Energy 40:383–391
Ding J, Zhong Q, Zhang S (2014) Catalytic efficiency of iron oxides in decomposition of H2O2 for simultaneous NOx and SOx removal: effect of calcinations temperature. J Mol Cata A: Chem 393:222–231
Khedr MH, Abdel Halim KS, Nasr MI, El-Mansy AM (2006) Effect of temperature on the catalytic oxidation of CO over nano-sized iron oxide. Mat Sci Eng A 430:40–45
Riley CR, Montgomery NE, Megally NN, Gunn JA, Davis LS (2012) Oxidation of cyclohexane by transition metal oxides on zeolites. Open Catal J 5:8–13
Nogueira FGE, Lopes JH, Silva AC, Lago RM, Fabris JD, Oliveria LCA (2011) Catalysts based on clay and iron oxide for oxidation of toluene. Appl Clay Sci 51:385–389
Tsodikov MV, Kugel VY, Slivinskii EV, Bondarenko GN, Maksimov YV, Alvarez MA, Hidalgo MC, Navio JA (2000) Selectivity and mechanism of cumene liquid-phase oxidation in the presence of powdered mixed iron-aluminum oxides prepared by alkoxy method. Appl Catal A: Gen 193:237–242
Ilisca E (1992) Ortho-para conversion of hydrogen molecules physisorbed on surfaces. Prog Surf Sci 41:217–335
Andrews L, Wang X (2004) Simple ortho-para hydrogen and para-ortho deuterium converter for matrix isolation spectroscopy. Rev Sci Instrum 75(9):3039–3044
Wu W, He Q, Jiang C (2008) Magnetic iron oxide nanoparticles: synthesis and surface fictionalization strategies. Nanoscale Res Lett 3:397–415
Abdulkadir I, Aliyu AB (2013) Some wet routes synthesis of hematite nanostructures. Afr J Pure Appl Chem 7(3):114–121
Wang F, Qin XF, Meng YF, Guo ZL, Yang LX, Ming YF (2013) Hydrothermal synthesis and characterization of α-Fe2O3 nanoparticles. Mater Sci Semicond Process 16:802–806
Galvita VV, Poelman H, Detavernier C, Marin GB (2015) Catalyst-assisted chemical looping for CO2 conversion to CO. Appl Catal B 164:184–191
Au-Yeung SCF, Denes G, Greedan JE, Eaton DR, Birchall T (1984) A novel synthesis route to “iron trihydroxide, Fe(OH)3”: characterization and magnetic properties. Inorg Chem 23:1513–1517
Mascolo MC, Pei Y, Ring TA (2013) Room temperature Co-precipitation synthesis of magnetite nanoparticles in a large pH window with different bases. Materials 6:5549–5567
Chaudhuri S, Bhattacharjya D, Yu J-S (2013) 1-dimensional porous α-Fe2O3 nanorods as high performance electrode material for supercapacitors. RSC Adv 3:25120–25128
Chung D-W, Kim TG (2007) Solvent effect on the hydrosilylation reactions for the synthesis of polydimethylsiloxane grafted with polyoxyethylene catalyzed by Speier’s catalyst. J Ind Eng Chem 13(6):979–984
Zhang Y, Liu Y, Yang G, Sun S, Tsubaki N (2007) Effect of impregnation solvent on Co/SiO2 catalyst for Fischer–Tropsch synthesis: a highly active and stable catalyst with bimodal sized cobalt particles. Appl Catal A 321:79–85
Kumar P, Singh RK, Rawat N, Barman PB, Katyal SC, Jang H, Lee H-N, Kumar R (2013) A novel method for controlled synthesis of nanosized hematite (α-Fe2O3) thin film on liquid-vapor interface. J Nanopart Res 15:1532
Eastoe J, Hollamby MJ, Hudson L (2006) Recent advances in nanoparticle synthesis with reversed micelles. Adv Coll Interface Sci 128–130:5–15
Zhang G-Y, Feng Y, Xu Y-Y, Gao D-Z, Sun Y-Q (2012) Controlled synthesis of mesoporous α-Fe2O3 nanorods and visible light photocatalytic property. Mater Res Bull 47:625–630
Xiao Z, Xia Y, Ren Z, Liu Z, Xu G, Chao C, Li X, Shen G, Han G (2012) Facile synthesis of single-crystalline mesoporous α-Fe2O3 and Fe3O4 nanorods as anode materials for lithium-ion batteries. J Mater Chem 22:20566–20573
Han L, Shan Z, Chen D, Yu X, Yang P, Tu B, Zhao D (2008) Mesoporous Fe2O3 microspheres: rapid and effective enrichment of phosphopeptides for MALDI-TOF MS analysis. J Colloid Interface Sci 318:315–321
Hassanjani-Roshan A, Reza Vaezi M, Shokuhfar A, Rajabali Z (2011) Synthesis of iron oxide nanoparticles via sonochemical method and their characterization. Particulogy 9:95–99
Sohn JR, Lim JS (2006) Catalytic properties of NiSO4/ZrO2 promoted with Fe2O3 for acid catalysis. Mater Res Bull 41:1225–1241
Routray K, Zhou W, Kiely CJ, Wachs IE (2011) Catalysis sicence of methanol oxidation over iron vanadate catalysts: nature of the catalytic active sites. ACS Catal 1:54–66
Spoto G, Vitillo JG, Cocina D, Damin A, Bonino F, Zecchina A (2007) FTIR spectroscopy and thermodynamics of hydrogen adsorbed in a cross-linked polymer. Phys Chem Chem Phys 9:4992–4999
Tam S, Fazardo ME (1999) Ortho/Para hydrogen converter for rapid deposition matrix isolation spectroscopy. Rev Sci Instrum 70(4):1926–1932
Dhanasekaran V, Anandhavelu S, Polychroniadis EK, Mahalingam T (2014) Microstructural properties evaluation of Fe2O3 nanostructures. Mater Lett 126:288–290
Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Khazal IG, Lopez-Serrano J, Williamson DC (2009) Reversible interactions with para-hydrogen enhanced NMR sensitivity by polarization transfer. Science 323:1708–1711
Wang C-T, Ro S-H (2006) Surface nature of nanoparticle gold/iron oxide aerogel catalysts. J Nano-Cryst Solids 352:35–43
Fujii T, de Groot FMF, Sawatzky GA, Vootgt FC, Hibma T, Okada K (1999) In situ XPS analysis of various iron oxides fills grown by NO2-assisted molecular-beam epitaxy. Physical Review B 59(4):3195–3202
Lin J-N, Chen J-H, Hsiao C-Y, Kang Y-M, Wan B-Z (2002) Gold supported on surface acidity modified Y-type and iron/Y-type zeolite for CO oxidation. Appl Catal B 36:19–29
Grosvenor AP, Kobe BA, Biesinger MC, Mcintyre NS (2004) Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf Interface Anal 36:1564–1574
Weckhuysen BW, Wang D, Rosynek MP, Lunsford JH (1998) Conversion of methane to benzene over transition metal ion ZSM-5 zeolites. J Catal 175:347–351
Epling WS, Hoflund GB, Weaver JF, Tsubota S, Haruta M (1996) Surface characterization study of Au/α-Fe2O3 and Au/Co3O4 low-temperature CO oxidation catalysts. J Phys Chem 100:9929–9934
Lin T-C, Seshadri G, Kelber JA (1997) A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl Surf Sci 119:83–92
Wu H, Wu G, Wang L (2015) Peculiar porous α-Fe2O3, γ-Fe2O3, and Fe3O4 nanospheres: facile synthesis and electromagnetic properties. Powder Technol 269:443–451
Hartl M, Gillis RC, Daemen L, Olds DP, Page K, Carlson S, Cheng Y, Hugle T, Iverson EB, Ramirez-Cuesta AJ, Lee Y, Muhrer G (2016) Hydrogen adsorption on two catalysts for the ortho- to parahydrogen conversion: Cr-doped silica and ferric oxide gel. Phys Chem Chem Phys 18:17281
Acknowledgement
This research work was supported by Convergence Research Program funded by the Ministry of Future Creation and Science (2013K000402), South Korea. J. Choi appreciates Hannam University for the supporting the period from April 1, 2017 to March 31, 2018. T. Das thanks to the Director and SRIC, IIT Roorkee for supporting through SMILE (SMILE-10).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Das, T., Choi, JG. & Oh, IH. Synthesis of Highly Effective α-Fe2O3 Catalyst for the Spin Conversion of Liquid Hydrogen. Proc. Natl. Acad. Sci., India, Sect. A Phys. Sci. 90, 399–409 (2020). https://doi.org/10.1007/s40010-019-00599-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40010-019-00599-3