Abstract
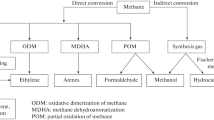
Different technologies for the synthesis of ethylene and propylene from natural gas are considered. The simplest ethylene synthesis method is the oxidative condensation (or dimerization) of methane (OCM process), but the implementation of this method is discouraged by a low ethylene yield. Among the relatively simple methods for the synthesis of ethylene (together with acetylene) immediately from methane are thermooxidative pyrolysis (TOP process) and pyrolysis in the presence of chlorine (Benson process), whose disadvantages are a high temperature and a great number of by-products. A wide range of processes for the synthesis of ethylene and propylene with a small amount of butylenes are based on the intermediate synthesis of syngas from methane and its further use for the direct synthesis of lower olefins or products for subsequent catalytic pyrolysis to lower olefins, such as methanol (methanol-to-olefins (MTO) and methanol-to-propylene (MTP) processes), dimethyl ether, and liquid fuel (Fischer–Tropsch method). The methyl chloride-to-olefins (MCTO) process consisting of the synthesis of methyl chloride via the oxidative chlorination of methane and the catalytic pyrolysis of methyl chloride differ from these three-stage processes in the absence of the syngas production stage. The direct oxidation of methane to methanol and the homologyzation of methanol to ethanol with further dehydration of ethanol to ethylene are also considered.
Similar content being viewed by others
References
Braginskii, O.B., Mirovoi neftegazovyi kompleks (World Oil and Gas Complex), Moscow: Nauka, 2004.
Ethylene. https://en.wikipedia.org/wiki/Ethylene
Braginskii, O.B., Nefte Gazo Khimiya, 2015, no. 2, p. 11.
Rynok etilena v Rossii (Market of Ethylene in Russia), market research report of the Academy of Conjuncture of Industrial Markets, Moscow: Nauka, 2006.
Propylene. https://en.wikipedia.org/wiki/Propene
Ochistka tekhnologicheskikh gazov (Purification of Process Gases), Semenova, T.A. and Leites, I.L., Eds., Moscow: Khimiya, 1977.
Spravochnik azotchika (Reference Book for Works with Nitrogen), Moscow: Khimiya, 1987.
Karavaev, M.M., Leonov, V.E., Popov, I.G., and Shepelev, E.G., Tekhnologiya sinteticheskogo metanola (Technology of Synthetic Methanol), Moscow: Khimiya, 1984.
Vinogradov A.S., Vorob’ev V.S., Daut V.A., Laurinaitis A., Sister V.G., Tauk, M.V., Chernyshev, A.K., and Bitkov, G.M., Sovremennoe sostoyanie proizvodstva metanola (State-of-Art of the Methanol Production), Moscow: Zh. Khimiya i Biznes, 2001.
Plate, N.A. and Slivinskii, E.V., Osnovy khimii i tekhnologii monomerov (Fundamentals of Chemistry and Technology of Monomers), Moscow: Nauka, 2002.
Mukhina, T.L., Barabanov, N.A., Babash, S.E., Men’shikov, V.A., and Avrekh, G.A., Piroliz uglevodorodnogo syr’ya (Pyrolysis of Hydrocarbons), Moscow: Khimiya, 1987.
Matar, S. and Hatch, L., Chemistry of Petrochemical Process, Boston: Gulf Professional, 2001.
Arutyunov, V.S. and Krylov, O.V., Okislitel’nye prevrashcheniya metana (Oxidative Conversion of Methane), Moscow: Nauka, 1998.
Dedov, A.G., Loktev, A.S., Parkhomenko, K.V., Moiseev, I.I., Men’shikov, V.A., and Filimonov, I.N., Khim. Prom-st. Segodnya, 2003, no. 3, p. 12.
Makhlin, V.A., Podlesnaya, M.V., Dedov, A.G., Loktev, A.S., Tel’pukhovskaya, N.O., and Moiseev, I.I., Ross. Khim. Zh., 2008, vol. 52, no. 5, p. 73.
Men’shikov, V.A., Lyakishev, G.G., Apel’baum, A.L., and Gol’dshtein, L.Kh., in Trudy Moskovskogo seminara po gazokhimii 2000–2002 (Proc. Moscow Symp. on Gas Chemistry 2000—2002), Vladimirov, A.I. and Lapidus, A.L., Eds., Moscow: Mosk. Inst. Neftekhim. Gazov. Prom-sti., 2003, p. 68.
Men’shikov, V.A. and Shamrai, O.B., Khim. Prom-st., 1998, no. 4, p. 6.
Ismailov, R.G., Promyshlennaya pererabotka nefti i razvitie neftekhimii (Industrial Processing of Oil and Development of Petrochemical Industry), Baku: Azerb. gos. izd., 1964.
Benson, S.W., US Patent 4199533, Chem. Abstr., 1980, vol. 93, 70984.
Treger, Yu.A. and Rozanov, V.N., Usp. khim., 1989, vol. 58, no. 1, p. 138.
Report of the Departement de Chimie Physique des Reactions, 7th Natural Gas Conversion Symposium, Dalian, China, 2004, abstr. no. 7-06-125.
Chambon, M., Marquaire, P.-M., and Côme, G.-M., C1 Mol. Chem., 1987, vol. 2, no. 1, p. 47.
Arutyunov, V.S., Katal. Prom-sti, 2003, no. 3, p. 3.
Arutyunov, V.S., Basevich, V.Ya., and Vedeneev, I.V., Usp. Khim., 1996, vol. 65, no. 3, p. 211.
Moiseev, I.I., Kinet. Catal., 2001, vol. 42, no. 1, p. 1.
Ullman’s Encyclopedia of Industrial Chemistry, Weinheim: VCH, 1986.
Rozovskii, A.Ya., Ross. Khim. Zh., 2003, vol. 47, no. 6, p. 53.
Report of Haldor Topsoe A/S, 7th Natural Gas Conversion Symposium, Dalian, China, 2004, abstr. no. 1-02-22.
Methanol, Topsoe Topics, November 1995.
Sosna, M.Kh., Doctoral (Tech.) Dissertation, Moscow, 1989.
Oil Gas J., 2002, vol. 100, no. 16, p. 64.
Sheldon, R.A., Chemicals from Synthesis Gas: Catalytic Reactions of CO and H2, Dordrecht: D. Reidel, 1983.
Kuznetsova, L.I., Nguen Kuang Guin’, Suzdorf, A.R., Beilin, L.A., Shpiro, E.S., and Minachev, Kh.M., Kinet. Katal., 1989, vol. 30, p. 944.
Xu, L., Wang, Q., Liang, D., Wang, X., Lin, L., Cui, W., and Yide, XuY., Appl. Catal., A, 1998, vol. 173, p. 19.
Modern trends in industrial catalysis (based on The European Congress on Catalysis), Katal. prom-sti, 2002, no. 1, p. 41; no. 3, p. 57.
Methanol (ICI low-pressure process), Hydrocarbon Process. (1966-2001), 1981, p. 183.
Neftegaz. Tekhnol., 2003, no. 5, p. 93.
Methanol—Haldor Topsoe A/S, Hydrocarbon Process. (1966-2001), 1981, p. 182.
Methanol: Lurgi low-pressure process), Hydrocarbon Process. (1966-2001), 1981, p. 184.
Streb, S., in Proc. World Congr. on Methanol, Copenhagen, 2000, p. 8.
Chen, J.Q., Vora, B.V., Fuglerud, T., and Kvisle, S., in Proc. 7th Natural Gas Conversion Symposium, Dalian, China, 2004, p. 6.
Chen, J.Q., Bozzano, A., Glover, B., Fuglerud, T., and Kvisle, S., Catal. Today, 2005, vol. 106, p. 103.
Marker, T.L., Vora, B.V., and Nil’sen, Kh.R., RF Patent 2165955, 2001.
Zhongmin, L., Coal-to-Olefin Technology in China, Dalian: Dalian Inst. Chem. Phys. Chin. Acad. Sci., 2009. zmldicp.ac.cn
Khadzhiev, S.N., Kolesnichenko, N.V., and Ezhova, N.N,. Pet. Chem., 2008, vol. 48, no. 5, p. 325.
Foley, T., Methanol to Olefins, Paper presented orally, September 23, 2007.
Methanol to Olefins, IOCL Conclave, February 7, 2014.
Koempel, H. and Liebner, W., in Natural Gas Conversion VIII: Proc. 8th Natural Gas Conversion Symp., Natal, Brazil, 2007.
Treger, Yu.A., Chagir, K.A., Savchenkov, S.V., and Repin, D.G., Gazov. Prom-st., 2015, no. 2, p. 17.
Coal and Natural Gas Chemical, Tecnon OrbiChem Marketing Seminar at APIC 2014, Pattaya, 2014.
Liu, Z., Sun, C., Wang, G., Wang, Q., and Cai, G., Fuel Process. Technol., 2000, vol. 62, p. 161.
Fujimoto, K., Shikada, T., Yamaoka, Y., and Sumigama, T., US Patent 5466720, 1995.
Rozovskii, A.Ya., Khim. Zhizn, 2002, no. 5, p. 8.
Kaiser, S.W., US Patent 4499327, 1985.
Shikada, T., Okhno, F., Ogava, E., Ono, M., Mizuguchi, M., Tomura, K., and Fudzhimoto, K., Kinet. Katal., 1990, vol. 40, no. 3, p. 440.
Rozanov, V.N., Treger, Yu.A., Murashova, O.P., Silina, I.S., and Averina, E.A., NefteGazoKhimiya, 2015, no. 2, p. 29.
Treger, Yu.A., Rozanov, V.N., Sokolova, S.V., and Murashova, O.P., Katal. Prom-sti., 2012, no. 3, p. 15.
Rozanov, V.N., Khim. Prom-st., 1996, no. 6, p. 21.
Treger, Yu.A., Rozanov, V.N., Trusov, L.I., Murashova, O.P., Yas’kova, V.Ya., and Silina, I.S., RF Patent 2522575, Byull. Izobret., 2014, no. 20.
Treger, Yu.A., Rozanov, V.N., and Flid, M.R., RF Patent 2394805, Byull. Izobret., 2010, no. 20.
Rozanov, V.N. and Treger, Yu.A., Katal. Prom-sti., 2015, vol. 15, no. 3, p. 49.
Kartashov, L.M., Rozanov, V.N., Treger, Yu.A., Flid, M.R., Kalyuzhnaya, T.L., and Tkach, D.V., Katal. Promsti., 2010, no. 3, p. 35.
Treger, Yu.A., Rozanov, V.N., Kartashov, L.M., Flid, M.R., Murashova, O.P., Averina, E.A., and Epikhina, S.V., RF Patent 2451005, 2012.
Treger, Yu.A., Rozanov, V.N., Lun’kov, S.A., Murashova, O.P., and Dasaeva, G.A., Katal. Prom-sti., 2009, no. 2, p. 14.
Treger, Yu.A., Rozanov, V.N., Dasaeva, G.S., Sokolova, S.V., Yas’kova, V., Trusov, L.I., Khadzhiev, S.N., Ivanova, I.I., and Knyazeva, E.E., RF Patent 2522576, Byull. Izobret., 2014, no. 20.
Olsbye, U., Saure, O.V., Muddada, N., Bordiga, S., Lamberti, C., Nilsen, M.N., Lillerud, K.P., and Svelle, S., Catal. Today, 2011, vol. 171, p. 211.
Sveiti, T.E., Neftegaz. Tekhnol., 2006, no. 1, p. 59.
Dry, M.E., Appl. Catal., A, 1996, vol. 138, p. 319.
Chemierohstoffe aus Kohle (Chemical Raw Materials from Coal), Falbe, J., Ed., Stuttgart: Georg Thieme, 1977.
Hammer, M., Joisten, S., Luengen, D., and Winkler, Int. J. Energy Res., 1994, vol. 18, p. 223.
Dry, M.E., Catal. Today, 1990, vol. 6, no. 3, p. 183.
Hydrocarbon Process. (1966-2001), 1981, p. 156.
Intille, G.M., in Proc. Chemrawn XVI Conference. SRI Consulting, 2003, p. 36.
Fox, J.M., Chen, T.P., Deden, B.D., Chem. End. Prog., 1990, vol. 86, p. 42.
Rozanov, V.N., Treger Yu.A., Razrabotka protsessov polucheniya khlormetana (Development of the processes of obtaining chlormethane), in Research works of Scientific institute “Synthesis”, Moscow, 1996.
Dong Xiang, Yu Qiau, Yi Mau, and Siyu Yang, Appl. Energy, 2014, vol. 113, p. 639.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Treger, Y.A., Rozanov, V.N. Technologies for the synthesis of ethylene and propylene from natural gas. Ref. J. Chem. 6, 83–123 (2016). https://doi.org/10.1134/S2079978016010039
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2079978016010039