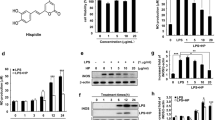
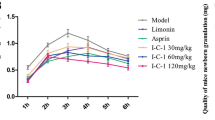
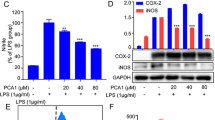
Abstract
Cordycepin, a natural derivative of adenosine, has been shown to exert pharmacological properties including anti-oxidation, antitumor, and immune regulation. It is reported that cordycepin is involved in the regulation of macrophage function. However, the effect of cordycepin on inflammatory cell infiltration in inflammation remains ambiguous. In this study, we investigated the potential role of cordycepin playing in macrophage function in CFA-induced inflammation mice model. In this model, we found that cordycepin prevented against macrophage infiltration in paw tissue and reduced interferon-γ (IFN-γ) production in both serum and paw tissue. Using luciferase reporter assay, we found that cordycepin suppressed IFN-γ-induced activators of transcription-1 (STAT1) transcriptional activity in a dose-dependent manner. Moreover, western blotting data demonstrated that cordycepin inhibited IFN-γ-induced STAT1 activation through attenuating STAT1 phosphorylation. Further investigations revealed that cordycepin inhibited the expressions of IFN-γ-inducible protein 10 (IP-10) and monokine induced by IFN-γ (Mig), which were the effector genes in IFN-γ-induced STAT1 signaling. Meanwhile, the excessive inflammatory cell infiltration in paw tissue was reduced by cordycepin. These findings demonstrate that cordycepin alleviates excessive inflammatory cell infiltration through down-regulation of macrophage IP-10 and Mig expressions via suppressing STAT1 phosphorylation. Thus, cordycepin may be a potential therapeutic approach to prevent and treat inflammation-associated diseases.








Similar content being viewed by others
References
Gai, G.Z., S.J. Jin, B. Wang, Y.Q. Li, and C.X. Li. 2004. The efficacy of Cordyceps militaris capsules in treatment of chronic bronchitis in comparison with Jinshuibao capsules. Chinese New Drugs Journal 13: 169–171.
Mamta, S. Mehrotra, V. Amitabh, P. Kirar, S. Vats, P. Nandi, P.S. Negi, and K. Misra. 2015. Phytochemical and antimicrobial activities of Himalayan Cordyceps sinensis (Berk.) Sacc. Indian Journal of Experimental Biology 53 (1): 36–43.
Ruma, I.M., E.W. Putranto, E. Kondo, R. Watanabe, K. Saito, Y. Inoue, K. Yamamoto, S. Nakata, M. Kaihata, H. Murata, and M. Sakaguchi. 2014. Extract of Cordyceps militaris inhibits angiogenesis and suppresses tumor growth of human malignant melanoma cells. International Journal of Oncology 45 (1): 209–218. https://doi.org/10.3892/ijo.2014.2397.
Choi, S.B., C.H. Park, M.K. Choi, D.W. Jun, and S. Park. 2004. Improvement of insulin resistance and insulin secretion by water extracts of Cordyceps militaris, Phellinus linteus, and Paecilomyces tenuipes in 90% pancreatectomized rats. Bioscience, Biotechnology, and Biochemistry 68 (11): 2257–2264. https://doi.org/10.1271/bbb.68.2257.
Cunningham, K.G., W. Manson, F.S. Spring, and S.A. Hutchinson. 1950. Cordycepin, a metabolic product isolated from cultures of Cordyceps militaris (Linn.) link. Nature 166 (4231): 949.
Hsu, Peng Yang, Yueh Hsin Lin, Erh Ling Yeh, Hui Chen Lo, Tai Hao Hsu, and Su. Che Chun. 2017. Cordycepin and a preparation from Cordyceps militaris inhibit malignant transformation and proliferation by decreasing EGFR and IL-17RA signaling in a murine oral cancer model. Oncotarget 8 (55): 93712–93728.
Sugar, A.M., and R.P. Mccaffrey. 1998. Antifungal activity of 3′-deoxyadenosine (cordycepin). Antimicrobial Agents and Chemotherapy 42 (6): 1424–1427.
Mueller, Werner E.G., Barbara E. Weiler, Ramamurthy Charubala, Wolfgang Pfleiderer, Leserman Lee, Robert W. Sobol, Robert J. Suhadolnik, and Heinz C. Schroeder. 1991. Cordycepin analogs of 2′,5′-oligoadenylate inhibit human immunodeficiency virus infection via inhibition of reverse transcriptase. Biochemistry 30 (8): 2027–2033.
Qing, R., Z. Huang, Y. Tang, Q. Xiang, and F. Yang. 2018. Cordycepin alleviates lipopolysaccharide-induced acute lung injury via Nrf2/HO-1 pathway. International Immunopharmacology 60: 18–25.
Tianzhu, Z., Y. Shihai, and D. Juan. 2015. The effects of Cordycepin on ovalbumin-induced allergic inflammation by strengthening Treg response and suppressing Th17 responses in ovalbumin-sensitized mice. Inflammation 38 (3): 1036–1043.
Choi, Y.H., G.Y. Kim, and H.H. Lee. 2014. Anti-inflammatory effects of cordycepin in lipopolysaccharide-stimulated RAW 264.7 macrophages through toll-like receptor 4-mediated suppression of mitogen-activated protein kinases and NF-kappaB signaling pathways. Drug Design, Development and Therapy 8: 1941–1953. https://doi.org/10.2147/DDDT.S71957.
Li, Y., K. Li, L. Mao, X. Han, K. Zhang, C. Zhao, and J. Zhao. 2016. Cordycepin inhibits LPS-induced inflammatory and matrix degradation in the intervertebral disc. PeerJ 4: e1992. https://doi.org/10.7717/peerj.1992.
Ren, Z., J. Cui, Z. Huo, J. Xue, H. Cui, B. Luo, L. Jiang, and R. Yang. 2012. Cordycepin suppresses TNF-alpha-induced NF-kappaB activation by reducing p65 transcriptional activity, inhibiting IkappaBalpha phosphorylation, and blocking IKKgamma ubiquitination. International Immunopharmacology 14 (4): 698–703. https://doi.org/10.1016/j.intimp.2012.10.008.
Martinez-Martinez, L., M.T. Martinez-Saavedra, P. Fuentes-Prior, M. Barnadas, M.V. Rubiales, J. Noda, I. Badell, C. Rodriguez-Gallego, and O. de la Calle-Martin. 2015. A novel gain-of-function STAT1 mutation resulting in basal phosphorylation of STAT1 and increased distal IFN-gamma-mediated responses in chronic mucocutaneous candidiasis. Molecular Immunology 68 (2 Pt C): 597–605. https://doi.org/10.1016/j.molimm.2015.09.014.
Rawlings, J.S., K.M. Rosler, and D.A. Harrison. 2004. The JAK/STAT signaling pathway. Journal of Cell Science 117 (Pt 8): 1281–1283. https://doi.org/10.1242/jcs.00963.
Park, O.J., M.K. Cho, C.H. Yun, and S.H. Han. 2015. Lipopolysaccharide of Aggregatibacter actinomycetemcomitans induces the expression of chemokines MCP-1, MIP-1alpha, and IP-10 via similar but distinct signaling pathways in murine macrophages. Immunobiology 220 (9): 1067–1074. https://doi.org/10.1016/j.imbio.2015.05.008.
Xu, Q., Y. Zhou, R. Zhang, Z. Sun, and L.F. Cheng. 2017. Antiarthritic activity of Qi-Wu rheumatism granule (a Chinese herbal compound) on complete Freund's adjuvant-induced arthritis in rats. Evidence-based Complementary and Alternative Medicine 2017: 1960517. https://doi.org/10.1155/2017/1960517.
Dong, L., J. Zhu, H. Du, H. Nong, X. He, and X. Chen. 2017. Astilbin from Smilax glabra Roxb. Attenuates inflammatory responses in complete Freund's adjuvant-induced arthritis rats. Evidence-based Complementary and Alternative Medicine 2017: 8246420. https://doi.org/10.1155/2017/8246420.
Robledo-Gonzalez, L.E., A. Martinez-Martinez, V.M. Vargas-Munoz, R.I. Acosta-Gonzalez, R. Plancarte-Sanchez, M. Anaya-Reyes, C. Fernandez Del Valle-Laisequilla, J.G. Reyes-Garcia, and J.M. Jimenez-Andrade. 2017. Repeated administration of mazindol reduces spontaneous pain-related behaviors without modifying bone density and microarchitecture in a mouse model of complete Freund's adjuvant-induced knee arthritis. Journal of Pain Research 10: 1777–1786. https://doi.org/10.2147/JPR.S136581.
Arango Duque, G., and A. Descoteaux. 2014. Macrophage cytokines: Involvement in immunity and infectious diseases. Frontiers in Immunology 5: 491. https://doi.org/10.3389/fimmu.2014.00491.
Hristodorov, D., R. Mladenov, M. Huhn, S. Barth, and T. Thepen. 2012. Macrophage-targeted therapy: CD64-based immunotoxins for treatment of chronic inflammatory diseases. Toxins (Basel) 4 (9): 676–694. https://doi.org/10.3390/toxins4090676.
Medzhitov, R. 2010. Inflammation 2010: New adventures of an old flame. Cell 140 (6): 771–776. https://doi.org/10.1016/j.cell.2010.03.006.
Malyshev, I.Y. 1900. Phenomena and signaling mechanisms of macrophage reprogramming. Patologicheskaia Fiziologiia i Eksperimentalnaia Terapiia 59 (2): 99–111.
Zhang, Da-wei, Zhen-lin Wang, Wei Qi, Wei Lei, and Guang-yue Zhao. 2014. Cordycepin (3′-deoxyadenosine) down-regulates the proinflammatory cytokines in inflammation-induced osteoporosis model. Inflammation 37 (4): 1044–1049.
Yarilina, Anna, Kai Xu, Chunhin Chan, and Lionel B. Ivashkiv. 2012. Regulation of inflammatory responses in tumor necrosis factor-activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis & Rheumatology 64 (12): 3856–3866.
Morrow, A.N., H. Schmeisser, T. Tsuno, and K.C. Zoon. 2011. A novel role for IFN-stimulated gene factor 3II in IFN-γ signaling and induction of antiviral activity in human cells. Journal of Immunology 186 (3): 1685–1693.
Yuan, S., T. Zheng, P. Li, R. Yang, J. Ruan, S. Huang, Z. Wu, and A. Xu. 2015. Characterization of Amphioxus IFN regulatory factor family reveals an archaic signaling framework for innate immune response. Journal of Immunology 195 (12): 5657–5666. https://doi.org/10.4049/jimmunol.1501927.
Huang, S.G., W.L. Guo, Z.C. Zhou, J.J. Li, F.B. Yang, and J. Wang. 2016. Altered expression levels of occludin, claudin-1 and myosin light chain kinase in the common bile duct of pediatric patients with pancreaticobiliary maljunction. BMC Gastroenterology 16: 7–7. https://doi.org/10.1186/s12876-016-0416-5.
Mady, H.H., and M.F. Melhem. 2002. FHIT protein expression and its relation to apoptosis, tumor histologic grade and prognosis in colorectal adenocarcinoma: An immunohistochemical and image analysis study. Clinical & Experimental Metastasis 19 (4): 351–358.
Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: A novel pathway of autocrine macrophage activation. The Journal of Experimental Medicine 187 (12): 2103–2108. https://doi.org/10.1084/jem.187.12.2103.
Liu, Q., Y.L. Zhang, W. Hu, S.P. Hu, Z. Zhang, X.H. Cai, and X.J. He. 2018. Transcriptome of porcine alveolar macrophages activated by interferon-gamma and lipopolysaccharide. Biochemical and Biophysical Research Communications 503 (4): 2666–2672. https://doi.org/10.1016/j.bbrc.2018.08.021.
Jaruga, B., F. Hong, W.H. Kim, and B. Gao. 2003. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: A critical role of IRF-1. Hepatology 38 (5): G1044.
Weiss, Günter, and Ulrich E. Schaible. 2015. Macrophage defense mechanisms against intracellular bacteria. Immunological Reviews 264 (1): 182–203.
Gregory, J.L., E.F. Morand, S.J. McKeown, J.A. Ralph, P. Hall, Y.H. Yang, S.R. McColl, and M.J. Hickey. 2006. Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. Journal of Immunology 177 (11): 8072–8079.
Gordon, S. 1998. The role of the macrophage in immune regulation. Research in Immunology 149 (7–8): 685–688.
Meda, L., M.A. Cassatella, G.I. Szendrei, L. Otvos Jr., P. Baron, M. Villalba, D. Ferrari, and F. Rossi. 1995. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature 374 (6523): 647–650. https://doi.org/10.1038/374647a0.
Dandona, P., A. Chaudhuri, and S. Dhindsa. 2010. Proinflammatory and prothrombotic effects of hypoglycemia. Diabetes Care 33 (7): 1686–1687. https://doi.org/10.2337/dc10-0503.
Li, Jin, Liping Zhong, Haibo Zhu, and Fengzhong Wang. 2017. The Protective Effect of Cordycepin on D-Galactosamine/Lipopolysaccharide-Induced Acute Liver Injury. Mediators of Inflammation,2017,(2017-3-28) 2017 (1): 3946706.
Zhang, D.W., Z.L. Wang, W. Qi, W. Lei, and G.Y. Zhao. 2014. Cordycepin (3′-deoxyadenosine) down-regulates the proinflammatory cytokines in inflammation-induced osteoporosis model. Inflammation 37 (4): 1044–1049. https://doi.org/10.1007/s10753-014-9827-z.
Lei, J., Y. Wei, P. Song, Y. Li, T. Zhang, Q. Feng, and G. Xu. 2018. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. European Journal of Pharmacology 818: 110–114. https://doi.org/10.1016/j.ejphar.2017.10.029.
Shin, S., S. Moon, Y. Park, J. Kwon, S. Lee, C.K. Lee, K. Cho, N.J. Ha, and K. Kim. 2009. Role of Cordycepin and adenosine on the phenotypic switch of macrophages via induced anti-inflammatory cytokines. Immune Network 9 (6): 255–264. https://doi.org/10.4110/in.2009.9.6.255.
Funding
This work was supported by the Guangxi Natural Science Foundation Program (2018GXNSFAA281211), the National Natural Science Foundation of China (81360312 and 81402306), and the Science and Technology Research Project of the Guangxi Colleges and Universities (YB2014080).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, R., Wang, X., Xi, D. et al. Cordycepin Attenuates IFN-γ-Induced Macrophage IP-10 and Mig Expressions by Inhibiting STAT1 Activity in CFA-Induced Inflammation Mice Model. Inflammation 43, 752–764 (2020). https://doi.org/10.1007/s10753-019-01162-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-01162-3