Abstract
Morphological and functional hierarchies occurring in contemporary biological entities are amalgamated via a small number of progressive key-steps termed as Major Transition in Evolution (MTE) that encompass steps of Major Transition in Individuality (MTI). Literature views MTE/MTI in nature as a sequential increase in complexity, and has contributed insights into the emergence of genuine MTI candidates that actually build higher order individuals from simpler entities and into their specific properties. The theory- By considering a novel MTI trajectory termed the ‘MTI continuum’ (independent of the tree of life that contemplates taxonomic correlations), I found no literature consensus for this continuum’s apex. Next, I consider the properties of biological entities termed as ‘superorganism’ (eusocial insects, humans), also considered as highly-developed MTIs. I classify ‘superorganism’ as being on the level of ‘miscellaneous transitions’ that have not yet developed into real MTIs and that do not meet the ‘individual’ physiognomy. Then I assign the emergence of three new MTI diachronic-classes, the colonial-organisms, chimerism and multi-chimerism, suggesting that they represent highly complex MTIs that belong at the apex of the MTI continuum. These novel MTIs are neither fraternal, nor egalitarian, deprived of ‘kinship’ and ‘fairness’ considerations, yet still generate genuine and distinct libertarian entities. I posit that these MTIs embody the qualities of real units of selection.

Similar content being viewed by others
References
Amar, K. O., Chadwick, N. E., & Rinkevich, B. (2008). Coral kin aggregations exhibit mixed allogeneic reactions and enhanced fitness during early ontogeny. BMC Evolutionary Biology, 8, 126.
Banfield, W. G., Woke, P. A., MacKay, C. M., & Cooper, H. L. (1965). Mosquito transmission of a reticulum cell sarcoma of hamsters. Science, 148, 1239–1240.
Barki, Y., Gateño, D., Graur, D., & Rinkevich, B. (2002). Soft-coral natural chimerism: A window in ontogeny allows the creation of entities comprised of incongruous parts. Marine Ecology Progress Series, 231, 91–99.
Barrett, S. C. (2008). Major evolutionary transitions in flowering plant reproduction: An overview. International Journal of Plant Sciences, 169, 1–5.
Bastiaans, E., Debets, A. J., & Aanen, D. K. (2015). Experimental demonstration of the benefits of somatic fusion and the consequences for allorecognition. Evolution, 69, 1091–1099.
Bayer, M. M., & Todd, C. D. (1997). Evidence for zooid senescence in the marine bryozoan Electra pilosa. Invertebrate Biology, 116, 331–340.
Boddy, A. M., Fortunato, A., Wilson Sayres, M., & Aktipis, A. (2015). Fetal microchimerism and maternal health: A review and evolutionary analysis of cooperation and conflict beyond the womb. BioEssays, 37, 1106–1118.
Buss, L. W. (1982). Somatic cell parasitism and the evolution of somatic tissue compatibility. Proceedings of the National Academy of Sciences of the United States of America, 79, 5337–5341.
Buss, L. W. (1983). Evolution, development, and the units of selection. Proceedings of the National Academy of Sciences of the United States of America, 80, 1387–1391.
Buss, L. W. (1987). The evolution of individuality. Princeton: Princeton University Press.
Casares, F. A., & Faugeron, S. (2016). Higher reproductive success for chimeras than solitary individuals in the kelp Lessonia spicata but no benefit for individual genotypes. Evolutionary Ecology, 30, 953–972.
Clarke, E. (2016). A levels-of-selection approach to evolutionary individuality. Biology and Philosophy, 31, 893–911.
Danchin, E., & Wagner, R. H. (1997). The evolution of coloniality: The emergence of new perspectives. Trends in Ecology & Evolution, 12, 342–347.
Das, U., & Das, A. K. (2000). Review of canine transmissible venereal sarcoma. Veterinary Research Communications, 24, 545–556.
Feldgarden, M., & Yund, P. O. (1992). Allorecognition in colonial marine invertebrates: Does selection favor fusion with kin or fusion with self? Biological Bulletin, 182, 155–158.
Folse, H. J., & Roughgarden, J. (2010). What is an individual organism? A multilevel perspective. Q Rev Biol, 85, 447–472.
Gilbert, S. F., Sapp, J., & Tauber, A. I. (2012). A symbiotic view of life: We have never been individuals. The Quarterly Review of Biology, 87, 325–341.
Gill, D. E., Chao, L., Perkins, S. L., & Wolf, J. B. (1995). Genetic mosaicism in plants and clonal animals. Annual Review of Ecology and Systematics, 26, 423–444.
Godfrey-Smith, P. (2016). Individuality, subjectivity, and minimal cognition. Biology and Philosophy, 31, 775–796.
Grosberg, R. K., & Strathmann, R. R. (2007). The evolution of multicellularity: A minor major transition? Annual Review of Ecology Evolution and Systematics, 38, 621–654.
Guay, A., & Pradeu, T. (2016). Individuals across the sciences. New York: Oxford University Press.
Haber, M. (2013). Colonies are individuals: revisiting the superorganism revival. In F. Bouchard & P. Huneman (Eds.), From groups to individuals: Evolution and emerging individuality (pp. 195–217). Cambridge: MIT Press.
Hamilton, A., Smith, N. R., & Haber, M. H. (2009). Social insects and the individuality thesis: Cohesion and the colony as a selectable individual. In J. Gadau & J. Fewell (Eds.), Organization of Insect Societies (pp. 572–589). Cambridge: Harvard University Press.
Hanschen, E. R., Davison, D. R., Grochau-Wright, Z. I., & Michod, R. E. (2017). Evolution of individuality: A case study in the volvocine green algae. Philosophy Theory and Practice in Biology, 9, 3.
Hanschen, E. R., Davison, D. R., Grochau-Wright, Z. I., & Michod, R. E. (2018). 20 individuality and the major evolutionary transitions. In S. B. Gissis, E. Lamm, & A. Shavit (Eds.), Landscapes of collectivity in the life sciences (pp. 255–268). Cambridge: MIT Press.
Hartikainen, H., Humphries, S., & Okamura, B. (2014). Form and metabolic scaling in colonial animals. Journal of Experimental Biology, 217, 779–786.
Harvell, C. D. (1994). The evolution of polymorphism in colonial invertebrates and social insects. The Quarterly Review of Biology, 69, 155–185.
Høeg, J. T., & Lutzen, J. (1995). Life cycle and reproduction in the Cirripedia Rhizocephala. Oceanography and Marine Biology: An Annual Review, 33, 427–485.
Jablonka, E., & Lamb, M. J. (2006). The evolution of information in the major transitions. Journal of Theoretical Biology, 239, 236–246.
Kennedy, P., Uller, T., & Helanterä, H. (2014). Are ant supercolonies crucibles of a new major transition in evolution? Journal of Evolutionary Biology, 27, 1784–1796.
Kikvidze, Z., & Callaway, R. M. (2009). Ecological facilitation may drive major evolutionary transitions. BioScience, 59, 399–404.
Magor, B. G., De Tomaso, A. W., Rinkevich, B., & Weissman, I. L. (1999). Allorecognition in colonial tunicates: Protection against predatory cell lineages? Immunological Reviews, 167, 69–79.
Mahmood, U., & O’Donoghue, K. (2014). Microchimeric fetal cells play a role in maternal wound healing after pregnancy. Chimerism, 5, 40–52.
Maynard, Smith J. (1988). Evolutionary progress and levels of selection. In M. H. Nitecki (Ed.), Evolutionary progress (pp. 219–230). Chicago: University of Chicago Press.
Maynard, Smith J., & Szathmáry, E. (1995). The major transitions in evolution. Oxford: Oxford University Press.
McShea, D. W., & Changizi, M. A. (2003). Three puzzles in hierarchical evolution. Integrative and Comparative Biology, 43(1), 74–81.
McShea, D. W., & Simpson, C. (2011). The miscellaneous transitions in evolution. In B. Calcott & K. Sterelny (Eds.), The major transitions in evolution revisited (pp. 17–33). Cambridge: MIT Press.
Metzger, M. J., Reinisch, C., Sherry, J., & Goff, S. P. (2015). Horizontal transmission of clonal cancer cells causes leukemia in soft-shell clams. Cell, 161, 255–263.
Metzger, M. J., et al. (2016). Widespread transmission of independent cancer lineages within multiple bivalve species. Nature, 534, 705–709.
Michod, R. E., & Nedelcu, A. M. (2003). On the reorganization of fitness during evolutionary transitions in individuality. Integrative and Comparative Biology, 43, 64–73.
Michod, R. E., Viossat, Y., Solari, C. A., Hurrand, M., & Nedelcu, A. M. (2006). Life history evolution and the origin of multicellularity. Journal of Theoretical Biology, 239, 257–272.
Mizrahi, D., Navarrete, S. A., & Flores, A. (2014). Groups travel further: Pelagic metamorphosis and polyp clustering allow higher dispersal potential in sun coral propagules. Coral Reefs, 33, 443–448.
Nelson, J. L., et al. (2007). Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet β cell microchimerism. Proceedings of the National academy of Sciences of the United States of America, 104, 1637–1642.
O’Malley, M. A., & Powell, R. (2016). Major problems in evolutionary transitions: How a metabolic perspective can enrich our understanding of macroevolution. Biology and Philosophy, 31, 159–189.
Pachut, J. F., Cuffey, R. J., & Anstey, R. L. (1991). The concepts of astogeny and ontogeny in stenolaemate bryozoans, and their illustration in colonies of Tabulipora carbonaria from the Lower Permian of Kansas. Journal of Paleontology, 65, 213–233.
Paz, G., & Rinkevich, B. (2002). Morphological consequences for multi-partner chimerism in Botrylloides, a colonial urochordate. Developmental and Comparative Immunology, 26, 615–622.
Pietsch, P. W. (2005). Dimorphism, parasitism, and sex revisited: Modes of reproduction among deep-sea ceratioid anglerfishes (Teleostei: Lophiiformes). Ichthyological Research, 52, 207–236.
Pilat, N., & Wekerle, T. (2010). Transplantation tolerance through mixed chimerism. Nature Reviews Nephrology, 6, 594–605.
Pineda-Krch, M., & Lehtila, K. (2004). Costs and benefits of genetic heterogeneity within organisms. Journal of Evolutionary Biology, 7, 1167–1177.
Popper, K. (1959). The Logic of Scientific Discovery. London: Routledge.
Pradeu, T. (2010). What is an organism? An immunological answer. History and Philosophy of the Life Sciences, 32, 247–268.
Queller, D. C. (1997). Cooperators since life began. The Quarterly Review of Biology, 72, 184–188.
Queller, D. C. (2000). Relatedness and the fraternal major transitions. Philosophical transactions of the Royal Society of London. Series B, 355, 1647–1655.
Rebbeck, C. A., Thomas, R., Breen, M., Leroi, A. M., & Burt, A. (2009). Origins and evolution of a transmissible cancer. Evolution, 63, 2340–2349.
Rietkerk, M., & Van de Koppel, J. (2008). Regular pattern formation in real ecosystems. Trends in Ecology & Evolution, 23, 169–175.
Rinkevich, B. (1996). Bi—vs. multi-chimerism in colonial urochordates: A hypothesis for links between natural tissue transplantation, allogenetics and evolutionary ecology. Experimental and Clinical Immunogenetics, 13, 61–69.
Rinkevich, B. (2000). A critical approach to the definition of Darwinian units of selection. Biological Bulletin, 199, 231–240.
Rinkevich, B. (2001). Human natural chimerism: An acquired character or a vestige of evolution? Human Immunol., 62, 651–657.
Rinkevich, B. (2002a). Germ cell parasitism as an ecological and evolutionary puzzle: Hitchhiking with positively selected genotypes. Oikos, 96, 25–30.
Rinkevich, B. (2002b). The colonial urochordate Botryllus schlosseri: From stem cells and natural tissue transplantation to issues in evolutionary ecology. BioEssays, 24, 730–740.
Rinkevich, B. (2004). Will two walk together, except they have agreed? Journal of Evolutionary Biology, 17, 1178–1179.
Rinkevich, B. (2005). Natural chimerism in colonial urochordates. Journal of Experimental Marine Biology and Ecology, 322, 93–109.
Rinkevich, B. (2011). Quo vadis chimerism? Chimerism, 2, 1–5.
Rinkevich, B. (2017). Senescence in modular animals- botryllid ascidians as a unique aging system. In R. Salguero-Gomez (Ed.), The Evolution of Senescence in the Tree of Life (pp. 220–237). Cambridge: Cambridge University Press.
Rinkevich, B. (2019). Coral chimerism as an evolutionary rescue mechanism to mitigate global climate change impacts. Global Change Biology, 25, 1198–1206.
Rinkevich, B., Shaish, L., Douek, J., & Ben-Shlomo, R. (2016). Venturing in coral larval chimerism: A compact functional domain with fostered genotypic diversity. Scientific Reports, 6, 19493.
Rinkevich, B., & Shapira, M. (1999). Multi-partner urochordate chimeras outperform two-partner chimerical entities. Oikos, 87, 315–320.
Rinkevich, B., & Weissman, I. L. (1987). Chimeras in colonial invertebrates: A synergistic symbiosis or somatic and germ-cell parasitism? Symbiosis, 4, 117–134.
Rinkevich, B., & Weissman, I. L. (1992). Chimeras vs genetically homogeneous individuals: Potential fitness costs and benefits. Oikos, 63, 119–124.
Rinkevich, B., & Yankelevich, I. (2004). Environmental split between germ cell parasitism and somatic cell synergism in chimeras of a colonial urochordate. Journal of Experimental Biology, 207, 3531–3536.
Roper, M., Ellison, C., Taylor, J. W., & Glass, N. L. (2011). Nuclear and genome dynamics in multinucleate ascomycete fungi. Current Biology, 21, R786–R793.
Santelices, B. (2004). Mosaicism and chimerism as components of intraorganismal genetic heterogeneity. Journal of Evolutionary Biology, 17, 1187–1188.
Santelices, B., Alvarado, J. L., & Flores, V. (2010). Size increments due to interindividual fusions: How much and for how long? Journal of Phycology, 46, 685–692.
Schultz, T. R., & Brady, S. G. (2008). Major evolutionary transitions in ant agriculture. Proceedings of the National academy of Sciences of the United States of America, 105, 5435–5440.
Shaish, L., Abelson, A., & Rinkevich, B. (2007). How plastic can phenotypic plasticity be? The branching coral Stylophora pistillata as a model system. PLoS ONE, 2(7), e644.
Siddle, H. V., & Kaufman, J. (2015). Immunology of naturally transmissible tumors. Immunology, 144, 11–20.
Simpson, C. (2011). How many levels are there? How insights from evolutionary transitions in individuality help measure the hierarchical complexity of life. In B. Calcott & K. Sterelney (Eds.), The major transitions in evolution revisited (pp. 199–226). Cambridge: MIT Press.
Stearns, S. C. (2007). Are we stalled part way through a major evolutionary transition from individual to group? Evolution, 61, 2275–2280.
Szathmáry, E. (2015). Toward major evolutionary transitions theory 2.0. Proceedings of the National Academy of Sciences of the United States of America, 112, 10104–10111.
Tivol, E., Komorowski, R., & Drobyski, W. R. (2005). Emergent autoimmunity in graft-versus-host disease. Blood, 105, 4885–4891.
Toh, T. C., & Chou, L. M. (2013). Aggregated settlement of Pocillopora damicornis planulae on injury sites may facilitate coral wound healing. Bulletin of Marine Science, 89, 583–584.
Wang, Y., et al. (2004). Fetal cells in mother rats contribute to the remodeling of liver and kidney after injury. Biochemical and Biophysical Research Communications, 325, 961–967.
West, S. A., Fisher, R. M., Gardner, A., & Kiers, E. T. (2015). Major evolutionary transitions in individuality. Proceedings of the National Academy of Sciences of the United States of America, 112, 10112–10119.
Wilson, D. S., & Sober, E. (1989). Reviving the superorganism. Journal of Theoretical Biology, 136, 337–356.
Wu, J., & Glass, N. L. (2001). Identification of specificity determinants and generation of alleles with novel specificity in the het-c heterokaryon incompatibility locus of Neurospora crassa. Molecular and Cellular Biology, 21, 1045–1057.
Acknowledgements
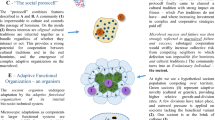
Thanks to G. Paz for drawing Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Rinkevich, B. The Apex Set-Up for the Major Transitions in Individuality. Evol Biol 46, 217–228 (2019). https://doi.org/10.1007/s11692-019-09481-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11692-019-09481-x