Abstract
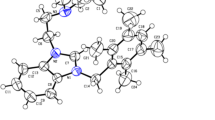
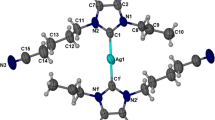
A series of bidendate 5,6-dimethyl benzimidazolium-based N-heterocyclic carbene (NHC) proligands and their silver complexes were synthesized. The synthetic approaches to the proligands were constrained by the methyl substituents, which impose a significant impact on the reactivity according to their sigma electron-donating abilities. The corresponding silver(I) complexes were obtained by in situ deprotonation of the NHCs and characterized by physicochemical and spectroscopic methods. In addition, a single-crystal X-ray diffraction study of complex C5 revealed its dinuclear structure. Irreversibility of redox events was observed in electrochemical studies of these complexes. In vitro anticancer studies of the azolium salts and their silver(I) complexes against human breast cancer (MDA-MB-231), colon cancer (HCT-116) and normal endothelial (EA.hy926) cells revealed that all the compounds are more cytotoxic to cancer cells than to normal cells and the complexes are more potent than their corresponding NHC proligands. Increased chain length, the presence of methyl substituents on the benzimidazole ring and aryl linker and two silver centres all enhance the biopotency of these complexes.
Graphical abstract
Substituted benzimidazolium-based N-heterocyclic carbene (NHC) proligands and silver complexes are advantageous anticancer tools for medicinal chemistry.








Similar content being viewed by others
References
Díez-González S (2016) N-heterocyclic carbenes: from laboratory to curiosities to efficient synthetic tools, vol 27. Royal Society of Chemistry, London
Hopkinson MN, Richter C, Schedler M, Glorius F (2014) An overview of N-heterocyclic carbenes. Nature 510:485
Mercs L, Albrecht M (2010) Beyond catalysis: N-heterocyclic carbene complexes as components for medicinal, luminescent, and functional materials applications. Chem Soc Rev 39:1903–1912
Al-Thagfi JR (2014) Synthesis, characterization and reactivity study of bis (imino)-N-heterocyclic carbene transition metal complexes. Ph.D. thesis, York University, Toronto, Canada
Haque RA, Hasanudin N, Hussein MA, Ahamed SA, Iqbal MA (2017) Bis-N-heterocyclic carbene silver (I) and palladium (II) complexes: efficient antiproliferative agents against breast cancer cells. Inorg Nano Metal Chem 47:131–137
Haque RA, Iqbal MA, Budagumpi S, Khadeer Ahamed MB, Abdul Majid AM, Hasanudin N (2013) Binuclear meta-xylyl-linked Ag (I)–N-heterocyclic carbene complexes of N-alkyl/aryl-alkyl-substituted bis-benzimidazolium salts: synthesis, crystal structures and in vitro anticancer studies. Appl Organomet Chem 27:214–223
Iqbal MA, Haque RA, Ahamed MBK, Majid AA, Al-Rawi SS (2013) Synthesis and anticancer activity of para-xylyl linked bis-benzimidazolium salts and respective Ag (I) N-heterocyclic carbene complexes. Med Chem Res 22:2455–2466
Iqbal MA, Haque RA, Budagumpi S, Ahamed MBK, Majid AMA (2013) Short metal–metal separations and in vitro anticancer studies of a new dinuclear silver (I)–N-heterocyclic carbene complex of para-xylyl-linked bis-benzimidazolium salt. Inorg Chem Commun 28:64–69
Iqbal MA, Umar MI, Haque RA, Ahamed MBK, Asmawi MZB, Majid AMSA (2015) Macrophage and colon tumor cells as targets for a binuclear silver (I) N-heterocyclic carbene complex, an anti-inflammatory and apoptosis mediator. J Inorg Biochem 146:1–13
Asif M, Iqbal MA, Hussein MA, Oon CE, Haque RA, Ahamed MBK, Majid ASA, Majid AMSA (2016) Human colon cancer targeted pro-apoptotic, anti-metastatic and cytostatic effects of binuclear silver (I)–N-heterocyclic carbene (NHC) complexes. Eur J Med Chem 108:177–187
Patil S, Deally A, Gleeson B, Hackenberg F, Müller-Bunz H, Paradisi F, Tacke M (2011) Synthesis, cytotoxicity and antibacterial studies of novel symmetrically and non-symmetrically p-nitrobenzyl-substituted N-heterocyclic carbene-silver (I) acetate complexes. Z anorganische allg Chem 637:386–396
Narasimhan B, Sharma D, Kumar P (2012) Benzimidazole: a medicinally important heterocyclic moiety. Med Chem Res 21:269–283
Catalano VJ, Malwitz MA (2003) Short metal–metal separations in a highly luminescent trimetallic Ag (I) complex stabilized by bridging NHC ligands. Inorg Chem 42:5483–5485
Catalano VJ, Etogo AO (2005) Luminescent coordination polymers with extended Au(I)–Ag (I) interactions supported by a pyridyl-substituted NHC ligand. J Organomet Chem 690:6041–6050
Nobili S, Mini E, Landini I, Gabbiani C, Casini A, Messori L (2010) Gold compounds as anticancer agents: chemistry, cellular pharmacology, and preclinical studies. Med Res Rev 30:550–580
Berners-Price SJ, Filipovska A (2011) Gold compounds as therapeutic agents for human diseases. Metallomics 3:863–873
Banti C, Giannoulis A, Kourkoumelis N, Owczarzak A, Poyraz M, Kubicki M, Charalabopoulos K, Hadjikakou S (2012) Mixed ligand–silver (I) complexes with anti-inflammatory agents which can bind to lipoxygenase and calf-thymus DNA, modulating their function and inducing apoptosis. Metallomics 4:545–560
Banti C, Kyros L, Geromichalos G, Kourkoumelis N, Kubicki M, Hadjikakou S (2014) A novel silver iodide metalo-drug: experimental and computational modelling assessment of its interaction with intracellular DNA, lipoxygenase and glutathione. Eur J Med Chem 77:388–399
Habib A, Vishkaei MN, Iqbal MA, Bhatti HN, Ahmed MK, Majid AA (2019) Unsymmetrically substituted benzimidazolium based silver (I)–N-heterocyclic carbene complexes: synthesis, characterization and in vitro anticancer study against human breast cancer and colon cancer. J Saudi Chem Soc. https://doi.org/10.1016/j.jscs.2019.03.002
Gümüşada R, Günay ME, Özdemir N, Çetinkaya B (2016) Bicyclic N-heterocyclic carbene (NHC) ligand precursors and their palladium complexes. J Coord Chem 69:1463–1472
Asekunowo PO, Haque RA (2014) Counterion-induced modulation in biochemical properties of nitrile functionalized silver (I)–N-heterocyclic carbene complexes. J Coord Chem 67:3649–3663
Zulikha HZ, Haque RA, Budagumpi S, Majid AMA (2014) Topology control in nitrile-functionalized silver (I)–N-heterocyclic carbene complexes: synthesis, molecular structures, and in vitro anticancer studies. Inorg Chim Acta 411:40–47
Karataş MO, Olgundeniz B, Günal S, Özdemir I, Alıcı B, Çetinkaya E (2016) Synthesis, characterization and antimicrobial activities of novel silver (I) complexes with coumarin substituted N-heterocyclic carbene ligands. Bioorg Med Chem 24:643–650
Kaplan HZ, Li B, Byers JA (2012) Synthesis and characterization of a bis (imino)-N-heterocyclic carbene analogue to bis (imino) pyridine iron complexes. Organometallics 31:7343–7350
Połczyński P, Jurczakowski R, Grochala W (2013) Strong and long-lived free-radical oxidizer based on silver (II). Mechanism of Ag (I) electrooxidation in concentrated H2SO4. J Phys Chem C 117:20689–20696
Gautier A, Cisnetti F (2012) Advances in metal–carbene complexes as potent anti-cancer agents. Metallomics 4:23–32
Liu W, Gust R (2013) Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem Soc Rev 42:755–773
Oehninger L, Rubbiani R, Ott I (2013) N-heterocyclic carbene metal complexes in medicinal chemistry. Dalton Trans 42:3269–3284
Jafari SF, Khadeer Ahamed MB, Iqbal MA, Al Suede FSR, Khalid SH, Haque RA, Nassar ZD, Umar MI, Abdul Majid AMS (2014) Increased aqueous solubility and proapoptotic activity of potassium koetjapate against human colorectal cancer cells. J Pharm Pharmacol 66:1394–1409
Kascatan-Nebioglu A, Panzner MJ, Tessier CA, Cannon CL, Youngs WJ (2007) N-heterocyclic carbene–silver complexes: a new class of antibiotics. Coord Chem Rev 251:884–895
Hartinger CG, Jakupec MA, Zorbas-Seifried S, Groessl M, Egger A, Berger W, Zorbas H, Dyson PJ, Keppler BK (2008) KP1019, a new redox-active anticancer agent—preclinical development and results of a clinical phase I study in tumor patients. Chem Biodivers 5:2140–2155
Snyder R, Hedli CC (1996) An overview of benzene metabolism. Environ Health Perspect 104:1165–1171
Friedberg EC, Walker GC, Siede W, Wood RD (2005) DNA repair and mutagenesis. American Society for Microbiology Press, Washington
Haque RA, Choo SY, Budagumpi S, Iqbal MA, Abdullah AAA (2015) Silver (I) complexes of mono-and bidentate N-heterocyclic carbene ligands: synthesis, crystal structures, and in vitro antibacterial and anticancer studies. Eur J Med Chem 90:82–92
Saint R (1999) 6.06 integration software for single crystal data. Bruker AXS Inc., Madison
Sheldrick G (2008) SADABS, Bruker area detector absorption corrections. Bruker AXS, Madison (based on method described in: Blessing RH. Acta Crystallogr A 51:33–38)
Release X (1997) 5.10, X-ray data preparation and reciprocal space exploration program. Bruker AXS Inc., Madison
Release S (1997) 5.10, The complete software package for single-crystal structure determination. Bruker AXS Inc., Madison
Sheldrick G (1997) SHELXS 97, program for the solution of crystal structure. University of Göttingen, Göttingen
Acknowledgements
Aqsa Habib would like to thank Higher Education Commission (HEC), Pakistan, for financial support during International Research Support Initiative Program (IRSIP) (1-8/HEC/HRD/2017/6940). She would also like to appreciate the efforts of Prof. Davit Zargarian (University de Montreal, Canada) for facilitating during research work in his laboratory during IRSIP period and Dr. Loic Mangin (University de Montreal, Canada) for solving crystal structure and helpful discussion for the preparation of this manuscript. Dr. Muhammad Adnan Iqbal is thankful to HEC for awarding research grant No. NRPU-8396 under National Research Program for Universities (NRPU).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Habib, A., Iqbal, M.A., Bhatti, H.N. et al. Effect of ring substitution on synthesis of benzimidazolium salts and their silver(I) complexes: characterization, electrochemical studies and evaluation of anticancer potential. Transit Met Chem 44, 431–443 (2019). https://doi.org/10.1007/s11243-019-00321-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-019-00321-7