Abstract
Aims/hypothesis
This randomised controlled trial was performed in India and the UK in people with prediabetes to study whether mobile phone short message service (SMS) text messages can be used to motivate and educate people to follow lifestyle modifications, to prevent type 2 diabetes.
Methods
The study was performed in people with prediabetes (n = 2062; control: n = 1031; intervention: n = 1031) defined by HbA1c ≥42 and ≤47 mmol/mol (≥6.0% and ≤6.4%). Participants were recruited from public and private sector organisations in India (men and women aged 35–55 years) and by the National Health Service (NHS) Health Checks programme in the UK (aged 40–74 years without pre-existing diabetes, cardiovascular disease or kidney disease). Allocation to the study groups was performed using a computer-generated sequence (1:1) in India and by stratified randomisation in permuted blocks in the UK. Investigators in both countries remained blinded throughout the study period. All participants received advice on a healthy lifestyle at baseline. The intervention group in addition received supportive text messages using mobile phone SMS messages 2–3 times per week. Participants were assessed at baseline and at 6, 12 and 24 months. The primary outcome was conversion to type 2 diabetes and secondary outcomes included anthropometry, biochemistry, dietary and physical activity changes, blood pressure and quality of life.
Results
At the 2 year follow-up (n = 2062; control: n = 1031; intervention: n = 1031), in the intention-to-treat population the HR for development of type 2 diabetes calculated using a discrete-time proportional hazards model was 0.89 (95% CI 0.74, 1.07; p = 0.22). There were no significant differences in the secondary outcomes.
Conclusions/interpretation
This trial in two countries with varied ethnic and cultural backgrounds showed no significant reduction in the progression to diabetes in 2 years by lifestyle modification using SMS messaging.
Trial registration
The primary study was registered on www.ClinicalTrials.gov (India, NCT01570946; UK, NCT01795833).
Funding
The study was funded jointly by the Indian Council for Medical Research and the UK Medical Research Council.
Similar content being viewed by others

Introduction
The public health challenge of type 2 diabetes is set to worsen as the prevalence rises from 425 million people globally in 2017 to 629 million by 2045 [1]. Diabetes is preceded by a period of intermediate hyperglycaemia (prediabetes), during which lifestyle interventions have been shown to reduce progression to diabetes in several RCTs [2,3,4].
The interventions in the initial diabetes prevention RCTs were labour intensive and difficult to scale up to reach large numbers of people at risk. Simple and scalable approaches to educate and motivate at-risk individuals to make behavioural changes using mobile phone short message service (SMS) text messages have been developed in several areas of preventive medicine [5,6,7,8,9,10,11]. In a previous RCT in India, we demonstrated that the delivery of a package of customised, tailored SMS messages based on the transtheoretical model (TTM) of behaviour change was effective compared with standard care, reducing the incidence of type 2 diabetes by 36% over 2 years [9]. In that RCT we recruited working Asian Indian men with persistent prediabetes defined as impaired glucose tolerance on two OGTTs. This method for defining prediabetes (and for assessing progression to diabetes) is time consuming for participants and the healthcare system and is difficult to scale up at societal level.
In the current study, we wished to test the generalisability of the results from the previous trial in India [9]. To do this, first, we included women as well as men; second, as the previous study was relatively small (537 participants), a larger number of participants was recruited; third, we tested the intervention in two ethnically and culturally different environments, India and the UK, using similar primary and secondary outcomes in both countries, with only minor differences reflecting the different populations and settings; finally, we used a more pragmatic method than glucose estimations to define hyperglycaemia, HbA1c, as recommended by the WHO [12]. The protocol permitted a comparative pooled analysis of outcomes from the two populations including an exploration of reasons for potential heterogeneity in the results.
Methods
Study design and participants
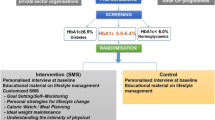
The detailed protocol has been reported previously [13]. In brief, a randomised, controlled clinical trial was conducted over 2 years in people with prediabetes defined by an HbA1c level of ≥42 and ≤47 mmol/mol (≥6.0% and ≤6.4%) (the high prediabetes range). Screening for possible participants took place in workplaces in India and at National Health Service (NHS) Health Checks and in primary care centres in the UK. All participants received structured education for prediabetes and the intervention group received, in addition, SMS messages about lifestyle 2–3 times weekly during the trial. Participants were monitored at baseline and at 6, 12 and 24 months, undertaking repeat assessment of HbA1c and blood glucose levels and completing questionnaires (the Euro quality of life 5 dimension 3 level [EQ-5D-3L], a recent physical activity questionnaire [RPAQ], a TTM of behavioural change questionnaire, and food frequency [UK] or 24 h dietary recall [India]). Physical activity (by accelerometer; ActiGraph GT3X+, ActiGraph, Pensacola, FL, USA) and acceptability of the SMS were monitored at baseline and during follow-up. The primary outcome was progression to diabetes. The secondary outcomes included anthropometric measurements, other cardiovascular risk factors and measures of lifestyle behaviours.
Figure 1 shows the flow diagram of pre-screening, screening, enrolment and randomisation, and the numbers of participants in the two countries. The total number of participants included in the analysis was 2062 (1031 in the control group, 1031 in the intervention group).
Pre-screening and screening
In India, pre-screening to identify people at high risk of developing diabetes was undertaken between April of 2012 and November of 2015 in Asian Indian men and women aged 35–55 years in Chennai and surrounding areas. The target population was employees from public and private sector organisations. Following a diabetes awareness programme, participants with no personal history of diabetes or other major physical or mental illness having three or more risk factors, including age 35–55 years, BMI ≥23 kg/m2, waist circumference ≥90 cm in men and ≥80 cm in women, first degree family history of type 2 diabetes, history of hypertension or prediabetes, or habitual sedentary behaviour, were selected for further screening using HbA1c [13]. Those with values in the high prediabetes range [14, 15] (≥42 and ≤47 mmol/mol [≥6.0% and ≤6.4%]) were invited to participate in the trial.
In the UK, pre-screening was conducted mainly using the NHS Health Checks programme, which is a cardiovascular and diabetes risk assessment offered routinely and free of charge to people aged 40–74 years without pre-existing diabetes, cardiovascular disease or kidney disease. The programme operates in primary care; people who met the HbA1c entry criteria (≥42 and ≤47 mmol/mol [≥6.0% and ≤6.4%]) were invited to participate in the trial if they fulfilled the other entry criteria. In some primary care centres, screening schemes other than the NHS Health Checks programme were used. Written, informed consent was obtained from all participants.
Randomisation and masking
Randomisation was performed in India using a computer-generated sequence, to either individually tailored mobile phone SMS messages supplementing baseline lifestyle advice (intervention group) or to a control group that received only the lifestyle modification advice at baseline (1:1). Randomisation in the UK was performed by a commercial organisation in random permuted blocks stratified by sex, age and BMI. Written, informed consent was obtained from the participants and in India permissions had also been obtained from the employers. In both countries, laboratory personnel and investigators were blinded to the participants’ group allocation until the end of the study. Staff involved in delivering the intervention and the participants themselves were, by necessity, not masked.
The study was registered on www.ClinicalTrials.gov (India, NCT01570946; UK, NCT01795833). The trial was registered separately in the two countries since the funding was received from two different national agencies.
Procedures for text messages
At baseline, in both countries, all trial participants received personalised education and motivation about healthy diet and the benefits of enhanced physical activity. In addition, the intervention group received regular SMS messages, typically 2–3 per week, to provide additional education and motivation. The content of the messages provided by SMS was similar in both countries. The messages used in the previous study in India [9] were modified and expanded. In the UK, a Patient and Public Involvement Group in the National Institute for Health Research Clinical Research Network provided input into the SMS message design and content. The messages provided tips, suggestions and positive reinforcement for healthy behaviours including goal setting, physical activity, dietary planning and personal strategies for lifestyle change. The message content was based on the TTM of behavioural change [16], a stage-based concept categorised by: precontemplation (not ready), contemplation (getting ready), preparation (ready), action and maintenance. We prepared an SMS database and grouped the messages to be appropriate for each TTM stage.
There were 75–80 messages in each TTM stage. Messages were sent to the participants based on the TTM staging performed at each follow-up. The type and content of the messages were changed frequently to avoid repetition. Messages were delivered by commercial service providers. In India, messages were in English and in two local languages and were sent between 06:30 hours and 08:30 hours or after 18:00 hours, as preferred by the participants. In the UK, messages were sent at 10:00 hours on alternate days. The preferred time was ascertained during the follow-up visits so that the messages did not go unnoticed.
Acceptability of SMS in the intervention group was assessed using a short questionnaire [9]. Responses to questions about message content, frequency, ease of understanding, whether messages were considered a disturbance and whether they were perceived as helpful in improving lifestyle were scored as 0 or 1. A total score of 6 was the most acceptable and 0 the least. A modified acceptability questionnaire was used in the UK.
Lifestyle and quality of life
Diet
At baseline, individualised dietary recommendations were delivered to balance food intake and physical activity, and to aim for an appropriate body weight. Advice included: avoidance of simple sugars and refined carbohydrates, reduction of total fat intake (<20 g/day) and inclusion of increased fibre-rich food (e.g. whole grains, legumes, vegetables and fruits). Evaluation was performed using 24 h dietary recall, a method used previously in India [9]. In the UK arm, a food frequency questionnaire was used for calculation of dietary energy intake and major food constituents [17], a method previously validated against 24 h dietary recall [18].
Physical activity
Participants who reported being sedentary or who undertook only light physical activity at baseline were advised to walk briskly every day for a minimum of 30 min. People who reported strenuous occupations or sufficient physical activity per day were advised to continue these activities. Physical activity was assessed by self-report using the RPAQ, which has previously been shown to provide a valid estimate of physical activity energy expenditure (PAEE), measured by the gold standard criterion method of doubly labelled water and time spent in different intensity levels [19]. We also assessed physical activity objectively using triaxial accelerometry (ActiGraph GT3X+) which has also been validated against criterion methods [20, 21].
Quality of life
The EQ-5D-3L version for India was administered to capture the individuals’ ‘perceived’ quality of life based on the effects of the health intervention [22].
The questionnaire consists of five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) and the responses record three levels of severity (no problems/some or moderate problems/extreme problems) within each dimension. The EQ-5D-3L summary measure was calculated using a value set derived from a UK sample since there are no published value sets in Indian populations.
Biochemical assessments
During the baseline and at each review, anthropometry, blood pressure (mean of two readings using sphygmomanometer), HbA1c and serum lipid profile (total cholesterol, low density lipoprotein, HDL-cholesterol and triacylglycerols) were measured using standard enzymatic procedures with quality control.
Ethics approvals
In India, the Ethics Review Committee of the India Diabetes Research Foundation and Dr. A. Ramachandran’s Diabetes Hospitals reviewed and approved the study protocol. An independent safety committee assessed study progress with unmasked data at 6 month intervals. In the UK, approval was from the Westminster Research Ethics Committee and site specific assessment (SSA) plus research and development (R&D) approvals were in place at each participating NHS Trust. Imperial College Academic Health Science Centre acted as the main sponsor. Delegated responsibilities were assigned to the participating NHS trusts.
Outcomes
In the UK, the primary outcome was progression to diabetes as defined by international criteria for fasting plasma glucose or HbA1c at any study review visit or in any healthcare setting. In India, information from study follow-up visits was available; thus, diabetes was defined on HbA1c alone.
The secondary outcomes were body weight and BMI, waist circumference, blood pressure, fasting plasma glucose, lipid levels, proportion achieving HbA1c ≤42 mmol/mol (≤6.0%), acceptability of SMS, dietary variables, physical activity and quality of life.
Statistical analyses
Based on results from the Indian Diabetes Prevention study [9], a 2 year risk of diabetes in the control group of 25% was assumed. With 2268 participants (1134 per group), the trial had 80% power to detect a relative reduction in risk of 20% as significant at the 5% level, allowing for approximately 4% withdrawals.
Baseline characteristics were summarised by randomised group using mean and standard deviation (continuous variables), median and interquartile range (continuous variables with a skewed distribution), or frequency and percentage (categorical variables).
The primary outcome was compared between intervention and control groups using a discrete-time proportional hazards model with a complementary log-log link function, since the data were interval censored [23], adjusted for country. The multiplicative interactions between randomised group and (1) country and (2) sex were tested using a Wald test. The HR for type 2 diabetes and 95% confidence intervals were reported for the overall trial population, and separately by country (UK/India) and sex, which were the only pre-specified subgroups.
Secondary outcomes, measured at specified time points during follow-up, were analysed using linear regression with random intercepts at the individual level to allow for repeated measures, including the baseline value of the outcome, country, randomised group and time, to estimate an overall intervention effect, and then also using randomised group × time interaction, to estimate intervention effects at each follow-up time. Accelerometer wear time was included in the model for objectively measured physical activity outcomes. Outcomes with a skewed distribution were log-transformed prior to analysis.
The trial was analysed on an intention-to-treat basis. The primary outcome was also analysed in a per-protocol population, which excluded individuals in whom the intervention was not successfully delivered. All analyses were pre-specified [13], and performed using Stata version 14.2 (Stata, College Station, TX, USA).
Results
Recruitment in India took place between 1 April 2012 and 1 November 2015, and in the UK between 1 June 2013 and 1 November 2017. Follow-up was for 2 years in both countries. The numbers assessed at pre-screening and screening are shown in Fig. 1. In the UK the recruitment took place in multiple primary care settings, mainly using the NHS Health Checks programme; routinely obtained data were scrutinised for eligibility and individuals were asked to participate if they fulfilled the entry criteria.
Primary and secondary outcomes
In total, 2062 participants were randomised (control: 1031; intervention: 1031). Baseline characteristics were similar in the two randomised groups (Table 1). The mean age was 52.0 (SD 10.3) years, and 64.0% of the participants overall were men.
During the 2 year follow-up period, 234 (22.7%) individuals in the control group and 216 (21.0%) in the intervention group developed diabetes. The cumulative percentage of individuals who developed diabetes at 6, 12 and 24 months in the control and intervention groups is shown in Fig. 2. There was no significant effect of the intervention on the primary outcome (HR for intervention vs control 0.89; 95% CI 0.74, 1.07; p = 0.22) (Fig. 3).
The overall intervention effects on the secondary outcomes are shown in Fig. 4 and in Table 2. Confidence intervals around the estimated effects were wide and overlapped zero. Mean values of most outcomes changed little between baseline and any of the follow-up visits in either randomised group.
Overall effect of intervention on secondary outcomes (n = 2062). Intervention effects represent differences between intervention and control groups, estimated from a linear regression model with random intercepts at the individual level, using measures of the outcome at all follow-up times, and including baseline value of the outcome, country, randomised group and time (months of follow-up). Intervention effects are presented in units of baseline SD of each outcome. Triacylglycerol results are presented using log-transformed values. MVPA, moderate-to-vigorous physical activity
For all of the secondary outcomes reported in Table 2, a maximum of 1.3% of individuals had missing values at baseline, except for work PAEE (22.6%), commuting PAEE (22.5%), each of the three ActiGraph physical activity measures (12.6%) and the EQ-5D-3L summary measure (41.1%). The percentages of individuals with missing values were similar in the two randomised groups. When estimating intervention effects using random intercepts linear regression, available data from all time points (including baseline) were included in the model. This assumes that any missing values at either baseline or another time point were missing at random.
Within this trial, the correlation between total physical activity (ActiGraph, counts/min) and total PAEE (kJ kg−1 day−1, RPAQ) was 0.28 at baseline and 0.32 at 24 months.
Although body weight did not change significantly, there were reductions in estimated intakes of total energy, fat, carbohydrates and protein, and an increase in estimated fibre intake, as assessed by a self-report questionnaire in each group, and as shown in Table 2.
The SMS acceptability questionnaire in India, where the median score out of 6 was 3, showed that messages were generally acceptable. Fewer than 5% of the participants reported that receiving the messages was a disturbance. In the UK the acceptability of SMS varied between 85% at 6 months to 82% at 24 months.
Over the 2 years of follow-up the observed percentages developing diabetes in both intervention and control groups were higher in India (control: 30.3%; intervention: 26.0%) than in the UK (control: 12.6%; intervention: 14.3%). There was no clear evidence of differential effects of the intervention by country or sex (tests of multiplicative interaction: randomised group × country: p = 0.33; randomised group × sex: p = 0.12) (Fig. 3).
Analysis of results per protocol in the intervention and control arms
In the per-protocol analysis, the overall results were similar to the intention-to-treat analysis for the primary outcome (HR for intervention vs control 0.95; 95% CI 0.79, 1.16; p = 0.63) and for the secondary outcomes.
Discussion
Although mobile technology is being widely applied in clinical management of a variety of long-term chronic disorders [6, 7, 10, 11, 24, 25] and also in modifying behaviour patterns such as smoking [26], the number of randomised intermediate and long-term studies in prevention of diabetes is limited. In this 2 year RCT involving 2062 participants with prediabetes recruited in two countries, India and the UK, with different ethnic and socio-cultural backgrounds, we have shown that delivery of a behavioural intervention by mobile technology is feasible. However, there was a non-significant risk reduction in the rate of progression of diabetes in 2 years by lifestyle modification using SMS messages.
A previous pilot trial of this intervention that we completed in India alone did find evidence of an effect. However, there are a number of differences between these trials that may explain the inconsistency in findings. First, the former study [9] was conducted in male employees of major industries, whereas the current study included men and women recruited from the general population. It is unlikely that it is the inclusion of women, rather than men alone, that has led to this inconsistency, since a pre-defined subgroup analysis provided weak evidence that the intervention may yield benefit in women. In other diabetes prevention studies, a major sex effect has not been observed [27]. It is more likely that the difference we observed is explained by the selected socio-cultural make-up of the population in the original study compared with the more population-based approach in the current study.
Second, the former study was conducted at a time when SMS messaging was novel in India and it is possible that this novelty and the fact that the messages appeared to come from a healthcare organisation may have influenced their effect. More recently, SMS messaging in India has proliferated, not only for the purpose of personal messaging, but also for mass advertising. Thus, it is possible that in the more recent study the ‘apparently personal’ targeted messaging that was previously effective is now just part of a slew of such messages, and thus may not only be diluted in effect but could also, by being of a similar nature to advertising, be considered an irritant.
Third, the way in which a high-risk prediabetes group was identified was fundamentally different. In the previous study we used the OGTT to define prediabetes and progression to diabetes. This test, and in particular the 2 h glucose, is highly variable and responsive to behaviour change [28], thus making it an excellent way to identify those at risk and the response to intervention. However, we sought not only to test the effectiveness of the intervention, but to do that in a way that was scalable. Unfortunately, the practicalities of the OGTT make it impossible to scale up to a mass intervention. Thus, we utilised the much more practical HbA1c test which could theoretically be employed in a real-life intervention programme to classify prediabetes and progression to diabetes. Although HbA1c has similar biological significance to other measures of glycaemia, for example, in terms of prediction of cardiovascular events [29], a possible disadvantage may be that, as an integrated measure of glucose control over a period of time, HbA1c is less sensitive to behaviour risk factor change, which may partially explain the lower estimate of effect size in this study. Finally, this study, unlike the former study, was conducted both in the UK and in India. It is possible that there could be country differences in the response to such an intervention, for socio-cultural or other reasons. The UK participants were recruited from primary care centres and thus, by definition, were in contact with an organised system of healthcare and would potentially have greater awareness of the importance of healthy lifestyle behaviours. By contrast, the participants in India are likely to have had less access to primary care and thus potentially a lower pre-existing awareness of health-promoting behaviours and a greater potential to benefit from this form of individual-level targeted prevention strategy.
Overall, the observed progression rate from prediabetes to diabetes was greater in India than in the UK. The rate in India is compatible with that in our previous Indian study [9] and the approximately 50% lower progression rate in the UK is consistent with other recent UK studies [30]. Previous analysis of the Diabetes Prevention Program (DPP) intervention within a single country has shown no differences in the risk of progression to diabetes from prediabetes between ethnic groups [31]. Our study shows that the rate of progression is markedly different between countries. We were not able in a pre-specified analysis to demonstrate significant differences in any intervention effect between the two countries, but this analysis may be under-powered to investigate differences in a low effect size. The low incidence of diabetes in the UK arm of this trial may have limited our ability to detect an effect of the intervention. Nor were we able to demonstrate significant differences in the secondary endpoints. Some of the apparent improvements that were observed in self-reported dietary components may be explained by reporting bias.
The results of this trial need to be set in the context of results of intervention evaluations elsewhere. In a recent trial in Denver, USA, Fischer et al evaluated text messaging as an aid to achieving weight loss in individuals with prediabetes [32]. Over 12 months, a clinically significant benefit in terms of weight loss was observed in the intervention group, but at 1 year HbA1c levels did not differ between the groups. A similar impact on weight was observed in a 12 month trial of a low-intensity lifestyle programme in Australian women [33], but although this intervention included monthly text messages on healthy behaviour, these messages were delivered in addition to phone coaching and provision of a programme manual, making it difficult to isolate the effect of the messages alone.
The utility of SMS in improving adherence to antiretroviral therapy [34] and smoking cessation has also been reported [35].
In a recent study in Bangladesh, a community-based intervention with facilitator-led group meetings was effective in preventing type 2 diabetes when an SMS-based intervention alone was not [36]. These studies and our own are compatible with the conclusions from a recent systematic review of electronically delivered weight loss programmes [37] that electronic delivery of lifestyle advice and motivation alone may be less effective than when supplemented with remote counselling or counselling in person. Cultural differences may also influence outcome and variability in results [24], although no major differences were observed in our study between effects in India and the UK.
Future studies should be powered to detect small intervention effects which may not be meaningful at an individual level, but which might be meaningful if scaled across a population level. We would also suggest that studies should be established to investigate more thoroughly the contextual factors that may influence the effectiveness of this type of intervention.
Data availability
The data that support the findings of this study are available from the corresponding author on request.
Abbreviations
- EQ-5D-3L:
-
Euro quality of life 5 dimension 3 level
- NHS:
-
National Health Service
- PAEE:
-
Physical activity energy expenditure
- RPAQ:
-
Recent physical activity questionnaire
- SMS:
-
Short message service
- TTM:
-
Transtheoretical model
References
International Diabetes Federation (2017) IDF Diabetes Atlas, 8th edition. IDF, Brussels Available from https://www.diabetesatlas.org/en/. Last accessed 20 Mar 2019
Ramachandran A, Snehalatha C, Shetty SA, Nanditha A (2014) Global health perspectives in prediabetes and diabetes prevention. In: Bergman M (ed) Primary prevention trials in type 2 diabetes. World Scientific Publishing, Singapore, pp 49–74
Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK (2018) Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care 41(7):1526–1534. https://doi.org/10.2337/dc17-2222
Ramachandran A, Snehalatha C, Shetty AS, Nanditha A (2013) Primary prevention of type 2 diabetes in South Asians—challenges and the way forward. Diabet Med 30(1):26–34. https://doi.org/10.1111/j.1464-5491.2012.03753.x
Fjeldsoe BS, Marshall AL, Miller YD (2009) Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med 36(2):165–173. https://doi.org/10.1016/j.amepre.2008.09.040
Cole-Lewis H, Kershaw T (2010) Text messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev 32:56–69. https://doi.org/10.1093/epirev/mxq004
WHO (2011) mHealth: new horizons for health through mobile technologies. Second global survey on eHealth. Available from https://www.who.int/goe/publications/goe_mhealth_web.pdf. Accessed 20 Apr 2016
Shetty AS, Chamukuttan S, Nanditha A, Raj RK, Ramachandran A (2011) Reinforcement of adherence to prescription recommendations in Asian Indian diabetes patients using short message service (SMS)—a pilot study. J Assoc Physicians India 59:711–714
Ramachandran A, Snehalatha C, Ram J et al (2013) Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomized controlled trial. Lancet Diabetes Endocrinol 1(3):191–198. https://doi.org/10.1016/S2213-8587(13)70067-6
Chow CK, Redfern J, Hillis GS et al (2015) Effect of lifestyle-focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. JAMA 314(12):1255–1263. https://doi.org/10.1001/jama.2015.10945
Thakkar J, Kurup R, Laba TL et al (2016) Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Intern Med 176(3):340–349. https://doi.org/10.1001/jamainternmed.2015.7667
Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Diabetes Res Clin Pract 93:299–309
Thomson H, Oliver N, Godsland IF et al (2018) Protocol for a clinical trial of text messaging in addition to standard care versus standard care alone in prevention of type 2 diabetes through lifestyle modification in India and the UK. BMC Endocr Disord 18(1):63. https://doi.org/10.1186/s12902-018-0293-8
National Institute for Health and Clinical Excellence (2012) Type 2 diabetes: prevention in people at high risk (PH38). Available from https://www.nice.org.uk/guidance/ph38. Last Accessed 14 May 2019
John WG, Hillson R, Alberti G (2011) Use of haemoglobin A1c (HbA1c) in the diagnosis of diabetes mellitus. The implementation of World Health Organization (WHO) guidance 2011. Pract Diab 29:12–12a. https://doi.org/10.1002/pdi.1648
Prochaska JO, Velicer WF (1997) The transtheoretical model of health behavior change. Am J Health Promot 12(1):38–48. https://doi.org/10.4278/0890-1171-12.1.38
Mulligan AA, Luben RN, Bhaniani A et al (2014) A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 4(3):e004503. https://doi.org/10.1136/bmjopen-2013-004503
Madden JP, Goodman SJ, Guthrie HA (1976) Validity of the 24-hr. recall. Analysis of data obtained from elderly subjects. J Am Diet Assoc 68(2):143–147
Besson H, Brage S, Jakes RW, Ekelund U, Wareham NJ (2010) Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults. Am J Clin Nutr 91(1):106–114. https://doi.org/10.3945/ajcn.2009.28432
White T, Westgate K, Hollidge S (2019) Estimating energy expenditure from wrist and thigh accelerometry in free-living adults: a doubly labelled water study. Int J Obes 43(11):2333–2342. https://doi.org/10.1038/s41366-019-0352-x
Corder K, Brage S, Ramachandran A, Snehalatha C, Wareham N, Ekelund U (2007) Comparison of two ActiGraph models for assessing free-living physical activity in Indian adolescents. J Sports Sci 25(14):1607–1611. https://doi.org/10.1080/02640410701283841
Rabin R, de Charro F (2001) EQ-5D: a measure of health status from the EuroQol group. Ann Med 33(5):337–343. https://doi.org/10.3109/07853890109002087
Prentice RL, Gloeckler LA (1978) Regression analysis of grouped survival data with application to breast cancer data. Biometrics 34(1):57–67
Müller AM, Alley S, Schoeppe S, Vandelanotte C (2016) The effectiveness of e-& mHealth interventions to promote physical activity and healthy diets in developing countries: a systematic review. Int J Behav Nutr Phys Act 13(1):109–114. https://doi.org/10.1186/s12966-016-0434-2
Grock S, Ku JH, Kim J, Moin T (2017) A review of technology-assisted interventions for diabetes prevention. Curr Diab Rep 17(11):107–112. https://doi.org/10.1007/s11892-017-0948-2
Scott-Sheldon LA, Lantini R, Jennings EG et al (2016) Text messaging-based interventions for smoking cessation: a systematic review and meta-analysis. JMIR Mhealth Uhealth 4(2):e49. https://doi.org/10.2196/mhealth.5436
Glechner A, Harreiter J, Gartlehner G et al (2015) Sex-specific differences in diabetes prevention: a systematic review and meta-analysis. Diabetologia 58(2):242–254. https://doi.org/10.1007/s00125-014-3439-x
Bartoli E, Fra GP, Carnevale Schianca GP (2011) The oral glucose tolerance test (OGTT) revisited. Eur J Intern Med 22(1):8–12. https://doi.org/10.1016/j.ejim.2010.07.008
Eeg-Olofsson K, Cederholm J, Nilsson PM et al (2010) New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). J Intern Med 268(5):471–482. https://doi.org/10.1111/j.1365-2796.2010.02265.x
Davies MJ, Gray LJ, Ahrabian D et al (2017) A community-based primary prevention programme for type 2 diabetes mellitus integrating identification and lifestyle intervention for prevention: a cluster randomised controlled trial. Programme Grants Appl Res 5(2). https://doi.org/10.1016/j.ypmed.2015.12.012
Knowler WC, Barrett-Connor E, Fowler SE et al (2002) The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346(6):393–403. https://doi.org/10.1056/NEJMoa012512
Fischer HH, Fischer IP, Pereira RI et al (2016) Text message support for weight loss in patients with prediabetes: a randomized clinical trial. Diabetes Care 39(8):1364–1370. https://doi.org/10.2337/dc15-2137
Lombard C, Harrison C, Kozica S, Zoungas S, Ranasinha S, Teede H (2016) Preventing weight gain in women in rural communities: a cluster randomized controlled trial. PLoS Med 13(1):e1001941. https://doi.org/10.1371/journal.pmed.1001941
Lester RT, Ritvo P, Mills EJ et al (2010) Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet 376(9755):1838–1845. https://doi.org/10.1016/S0140-6736(10)61997-6
Free C, Knight R, Robertson S et al (2011) Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet 378(9785):49–55. https://doi.org/10.1016/S0140-6736(11)60701-0
Fottrell E, Ahmed N, Morrison J et al (2019) Community groups or mobile phone messaging to prevent and control type 2 diabetes and intermediate hyperglycaemia in Bangladesh (DMagic): a cluster-randomised controlled trial. Lancet Diabetes Endocrinol 7(3):200–212. https://doi.org/10.1016/S2213-8587(19)30001-4
Joiner KL, Nam S, Whittemore R (2017) Lifestyle interventions based on the diabetes prevention program delivered via eHealth: a systematic review and meta-analysis. Prev Med 100:194–207. https://doi.org/10.1016/j.ypmed.2017.04.033
Acknowledgements
We thank the Indian Council for Medical Research and the UK Medical Research Council for funding this study. Imperial College London is grateful for support from the NW London National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research & Care. Most of the recruitment in the UK was performed by members of the NIHR Clinical Research Network. The authors of the Indian arm acknowledge the excellent help rendered by the epidemiology team of India Diabetes Research Foundation, C.K. Sathish Kumar and M. Karthikeyan. The secretarial and statistical assistance of L. Vijaya is acknowledged. We thank K. Raghavan, N. Murugesan and C.R.A. Moses for serving as members of the data safety and monitoring committee. We also thank the management departments of various service organisations. We are particularly indebted to all of the participants of the study for their co-operation.
Funding
Funding was obtained jointly from the Indian Council for Medical Research (ref. no. 58/1/6/ICMR-MRC/2009-NCD-II) and the UK Medical Research Council (joint funding ref.: MR/J000183/1). NJW, SJS, SB and KW are supported by the Medical Research Council (MC_UU_12015/1 and MC_UU_12015/3). In the UK, fieldwork was conducted by National Institute for Health Research Clinical Research Network (NIHR CRN) staff. The Indian Council of Medical Research, the Medical Research Council Joint Initiative: Chronic Non-Communicable Diseases Research and the NIHR CRN had no role in study design, data collection, data analysis, data interpretation or writing of the report.
Author information
Authors and Affiliations
Contributions
ARam, DGJ and NJW are the chief investigators of this trial. All authors contributed to the study design, developing the protocol, supervising study progress and drafting the manuscript or revising it with critical input. HT managed and coordinated the conduct of all aspects of the study in the UK. PS, MS and KS participated in and coordinated the Indian fieldwork and data collection. ARam, NJW, CS, AN, IFG, WS, SJS, KW and SB contributed to the data analysis plan and preparing the data for analysis. WS, SJS, KW and SB contributed to data preparation and statistical analysis. All authors have read and approved the final draft. ARam is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nanditha, A., Thomson, H., Susairaj, P. et al. A pragmatic and scalable strategy using mobile technology to promote sustained lifestyle changes to prevent type 2 diabetes in India and the UK: a randomised controlled trial. Diabetologia 63, 486–496 (2020). https://doi.org/10.1007/s00125-019-05061-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-019-05061-y