Abstract
Purpose
Gamma Knife radiosurgery (GKRS) is a non-invasive procedure for the treatment of brain metastases. This study sought to determine whether radiomic features of brain metastases derived from pre-GKRS magnetic resonance imaging (MRI) could be used in conjunction with clinical variables to predict the effectiveness of GKRS in achieving local tumor control.
Methods
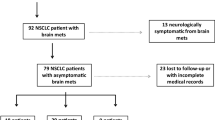
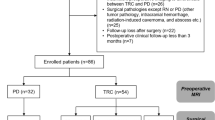
We retrospectively analyzed 161 patients with non-small cell lung cancer (576 brain metastases) who underwent GKRS for brain metastases. The database included clinical data and pre-GKRS MRI. Brain metastases were demarcated by experienced neurosurgeons, and radiomic features of each brain metastasis were extracted. Consensus clustering was used for feature selection. Cox proportional hazards models and cause-specific proportional hazards models were used to correlate clinical variables and radiomic features with local control of brain metastases after GKRS.
Results
Multivariate Cox proportional hazards model revealed that higher zone percentage (hazard ratio, HR 0.712; P = .022) was independently associated with superior local tumor control. Similarly, multivariate cause-specific proportional hazards model revealed that higher zone percentage (HR 0.699; P = .014) was independently associated with superior local tumor control.
Conclusions
The zone percentage of brain metastases, a radiomic feature derived from pre-GKRS contrast-enhanced T1-weighted MRIs, was found to be an independent prognostic factor of local tumor control following GKRS in patients with non-small cell lung cancer and brain metastases. Radiomic features indicate the biological basis and characteristics of tumors and could potentially be used as surrogate biomarkers for predicting tumor prognosis following GKRS.



Similar content being viewed by others
References
Kaal ECA, Niël CGJH, Vecht CJ (2005) Therapeutic management of brain metastasis. Lancet Neurol 4:289–298. https://doi.org/10.1016/s1474-4422(05)70072-7
Schouten LJ, Rutten J, Huveneers HA, Twijnstra A (2002) Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 94:2698–2705. https://doi.org/10.1002/cncr.10541
Schuette W (2004) Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 45:S253–S257
Sørensen J, Hansen H, Hansen M, Dombernowsky P (1988) Brain metastases in adenocarcinoma of the lung: frequency, risk groups, and prognosis. J Clin Oncol 6:1474–1480
Ali A, Goffin JR, Arnold A, Ellis PM (2013) Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. https://doi.org/10.3747/co.20.1481
Hussain A, Brown PD, Stafford SL, Pollock BE (2007) Stereotactic radiosurgery for brainstem metastases: Survival, tumor control, and patient outcomes. Int J Radiat Oncol Biol Phys 67:521–524. https://doi.org/10.1016/j.ijrobp.2006.08.081
Trifiletti DM, Lee C-C, Kano H, Cohen J, Janopaul-Naylor J, Alonso-Basanta M, Lee JYK, Simonova G, Liscak R, Wolf A, Kvint S, Grills IS, Johnson M, Liu K-D, Lin C-J, Mathieu D, Héroux F, Silva D, Sharma M, Cifarelli CP, Watson CN, Hack JD, Golfinos JG, Kondziolka D, Barnett G, Lunsford LD, Sheehan JP (2016) Stereotactic radiosurgery for brainstem metastases: an international cooperative study to define response and toxicity. Int J Radiat Oncol Biol Phys 96:280–288. https://doi.org/10.1016/j.ijrobp.2016.06.009
Jawahar A, Matthew RE, Minagar A, Shukla D, Zhang JH, Willis BK, Ampil F, Nanda A (2004) Gamma knife surgery in the management of brain metastases from lung carcinoma: a retrospective analysis of survival, local tumor control, and freedom from new brain metastasis. J Neurosurg 100:842–847
Petrovich ZYC, Giannotta SL, O'Day S, Apuzzo ML (2002) Survival and pattern of failure in brain metastasis treated with stereotactic gamma knife radiosurgery. J Neurosurg 97(Supplement 5):499–506
Ayala-Peacock DN, Peiffer AM, Lucas JT, Isom S, Kuremsky JG, Urbanic JJ, Bourland JD, Laxton AW, Tatter SB, Shaw EG, Chan MD (2014) A nomogram for predicting distant brain failure in patients treated with gamma knife stereotactic radiosurgery without whole brain radiotherapy. Neuro Oncol 16:1283–1288. https://doi.org/10.1093/neuonc/nou018
Zindler JD, Slotman BJ, Lagerwaard FJ (2014) Patterns of distant brain recurrences after radiosurgery alone for newly diagnosed brain metastases: Implications for salvage therapy. Radiother Oncol 112:212–216. https://doi.org/10.1016/j.radonc.2014.07.007
Gorovets D, Ayala-Peacock D, Tybor DJ, Rava P, Ebner D, Cielo D, Noren G, Wazer DE, Chan M, Hepel JT (2017) Multi-institutional nomogram predicting survival free from salvage whole brain radiation after radiosurgery in patients with brain metastases. Int J Radiat Oncol Biol Phys 97:246–253. https://doi.org/10.1016/j.ijrobp.2016.09.043
McTyre E, Ayala-Peacock D, Contessa J, Corso C, Chiang V, Chung C, Fiveash J, Ahluwalia M, Kotecha R, Chao S, Attia A, Henson A, Hepel J, Braunstein S, Chan M (2018) Multi-institutional competing risks analysis of distant brain failure and salvage patterns after upfront radiosurgery without whole brain radiotherapy for brain metastasis. Ann Oncol 29:497–503. https://doi.org/10.1093/annonc/mdx740
Baschnagel AM, Meyer KD, Chen PY, Krauss DJ, Olson RE, Pieper DR, Maitz AH, Ye H, Grills IS (2013) Tumor volume as a predictor of survival and local control in patients with brain metastases treated with Gamma Knife surgery. J Neurosurg 119:1139–1144. https://doi.org/10.3171/2013.7.JNS13431
Kim DG, Chung HT, Gwak HS, Paek SH, Jung HW, Han DH. (2000) Gamma knife radiosurgery for brain metastases: prognostic factors for survival and local control. J Neurosurg 93(supplement_3): 23–29
Lee H-L, Chung T-S, Ting L-L, Tsai J-T, Chen S-W, Chiou J-F, Leung HW-C, Liu HE (2012) EGFR mutations are associated with favorable intracranial response and progression-free survival following brain irradiation in non-small cell lung cancer patients with brain metastases. Radiat Oncol 7:181
Lee C-C, Hsu SP, Lin C-J, Wu H-M, Chen Y-W, Luo Y-H, Chiang C-L, Hu Y-S, Chung W-Y, Shiau C-Y (2019) Epidermal growth factor receptor mutations: association with favorable local tumor control following Gamma Knife radiosurgery in patients with non–small cell lung cancer and brain metastases. J Neurosurg 1:1–8
Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, Forster K, Aerts HJ, Dekker A, Fenstermacher D, Goldgof DB, Hall LO, Lambin P, Balagurunathan Y, Gatenby RA, Gillies RJ (2012) Radiomics: the process and the challenges. Magn Reson Imaging 30:1234–1248. https://doi.org/10.1016/j.mri.2012.06.010
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446. https://doi.org/10.1016/j.ejca.2011.11.036
Gillies RJ, Kinahan PE, Hricak H (2015) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, Bussink J, Monshouwer R, Haibe-Kains B, Rietveld D, Hoebers F, Rietbergen MM, Leemans CR, Dekker A, Quackenbush J, Gillies RJ, Lambin P (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006. https://doi.org/10.1038/ncomms5006
Li Z-C, Li Q-H, Song B-L, Chen Y-S, Sun Q-C, Xie Y-Q, Wang L (2016) Clustering of MRI radiomics features for glioblastoma multiforme: an initial study. International Conference on Medical Imaging and Augmented Reality. Springer, Berlin, pp 311–319
Kniep HC, Madesta F, Schneider T, Hanning U, Schonfeld MH, Schon G, Fiehler J, Gauer T, Werner R, Gellissen S (2019) Radiomics of brain MRI: utility in prediction of metastatic tumor type. Radiology 290:479–487. https://doi.org/10.1148/radiol.2018180946
Lee C-C, Chou C-L, Chen C-J, Yang H-C, Wu H-M, Shiau C-Y, Pan DH-C, Chung W-Y (2018) Stereotactic radiosurgery for hypervascular intracranial tumors. J Neuro Oncol 140:547–558. https://doi.org/10.1007/s11060-018-2980-8
Lee CC, Pan DH, Chung WY, Liu KD, Yang HC, Wu HM, Guo WY, Shih YH (2012) Brainstem cavernous malformations: the role of Gamma Knife surgery. J Neurosurg. https://doi.org/10.3171/2012.8.GKS121066
Lee CC, Wintermark M, Xu Z, Yen CP, Schlesinger D, Sheehan JP (2014) Application of diffusion-weighted magnetic resonance imaging to predict the intracranial metastatic tumor response to gamma knife radiosurgery. J Neurooncol 118:351–361. https://doi.org/10.1007/s11060-014-1439-9
Lee CC, Yen CP, Xu Z, Schlesinger D, Sheehan J (2014) Large intracranial metastatic tumors treated by Gamma Knife surgery: outcomes and prognostic factors. J Neurosurg 120:52–59. https://doi.org/10.3171/2013.9.JNS131163
Pai FY, Chen CJ, Wang WH, Yang HC, Lin CJ, Wu HM, Lin YC, Chen HS, Yen YS, Chung WY, Guo WY, Pan DH, Shiau CY, Lee CC (2019) Low-dose Gamma Knife radiosurgery for acromegaly. Neurosurgery 85:E20–E30. https://doi.org/10.1093/neuros/nyy410
Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, Werner-Wasik M, Demas W, Ryu J, Bahary J-P, Souhami L, Rotman M, Mehta MP, Curran WJ (2004) Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 363:1665–1672. https://doi.org/10.1016/s0140-6736(04)16250-8
Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, Kenjyo M, Oya N, Hirota S, Shioura H (2006) Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 295:2483–2491
Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, Farnan N (2000) Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90–05. International J Radiat Oncol Biol Phys 47:291–298
van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, Beets-Tan RGH, Fillion-Robin JC, Pieper S, Aerts H (2017) Computational radiomics system to decode the radiographic phenotype. Cancer Res 77:e104–e107. https://doi.org/10.1158/0008-5472.CAN-17-0339
Rizzo S, Botta F, Raimondi S, Origgi D, Fanciullo C, Morganti AG, Bellomi M (2018) Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp 2:36. https://doi.org/10.1186/s41747-018-0068-z
Parmar C, Leijenaar RT, Grossmann P, Rios Velazquez E, Bussink J, Rietveld D, Rietbergen MM, Haibe-Kains B, Lambin P, Aerts HJ (2015) Radiomic feature clusters and prognostic signatures specific for Lung and Head & Neck cancer. Sci Rep 5:11044. https://doi.org/10.1038/srep11044
Braman NM, Etesami M, Prasanna P, Dubchuk C, Gilmore H, Tiwari P, Plecha D, Madabhushi A (2017) Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Res. https://doi.org/10.1186/s13058-017-0846-1
Monti S, Tamayo P, Mesirov J, Golub T (2003) Consensus clustering: a resampling-based method for class discovery and visualization of gene expression microarray data. Mach Learn 52:91–118
Snell JW, Sheehan J, Stroila M, Steiner L (2006) Assessment of imaging studies used with radiosurgery: a volumetric algorithm and an estimation of its error. J Neurosurg 104:157–162
Dignam JJ, Zhang Q, Kocherginsky M (2012) The use and interpretation of competing risks regression models. Clin Cancer Res 18:2301–2308
Noordzij M, Leffondré K, van Stralen KJ, Zoccali C, Dekker FW, Jager KJ (2013) When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 28:2670–2677
Zhang Z (2017) Survival analysis in the presence of competing risks. Ann Transl Med 5(3):47
Therneau TM, Grambsch PM (2013) Modeling survival data: extending the Cox model. Springer Science & Business Media, Berlin
Peterson AM, Meltzer CC, Evanson EJ, Flickinger JC, Kondziolka D (1999) MR imaging response of brain metastases after gamma knife stereotactic radiosurgery. Radiology 211:807–814
Martens K, Meyners T, Rades D, Tronnier V, Bonsanto MM, Petersen D, Dunst J, Dellas K (2013) The prognostic value of tumor necrosis in patients undergoing stereotactic radiosurgery of brain metastases. Radiat Oncol 8:162
Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu VT (2009) Hypoxia and radiation therapy: past history, ongoing research, and future promise. Curr Mol Med 9:442–458
Kuhnt T, Mueller A-C, Pelz T, Haensgen G, Bloching M, Koesling S, Schubert J, Dunst J (2005) Impact of tumor control and presence of visible necrosis in head and neck cancer patients treated with radiotherapy or radiochemotherapy. J Cancer Res Clin Oncol 131:758–764
Stadler P, Becker A, Feldmann HJ, Hänsgen G, Dunst J, Würschmidt F, Molls M (1999) Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. Int J Radiat Oncol Biol Phys 44:749–754
Acknowledgements
The authors would like to thank all colleagues who contributed to this study. We are grateful to our research assistants, Fong-Jiao Lee, Hsueh-Jen Huang, Wen-Chi Ku, Yi-Bei Tseng, and Jr Lan Huang for their data recording and transcription. We thank the editor and series editor for constructive criticisms of an earlier version of this article. This work was financially supported in part by the Ministry of Science and Technology, Taiwan, under the project MOST 108-2221-E-038-019 and the project MOST 108-2634-F-010-002, in part by the Research Grants for Newly Hired Faculty by the Taipei Medical University in Taiwan, under the project TMU 108-AE1-B04, and in part by the Brain Research Center, National Yang-Ming University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material. Supplementary Fig.1 Consensus clustering delta area plot. The relative change in the area under cumulative distribution function curves (y-axis) approximately converges to minimum at 8 or more clusters (x-axis). Therefore, we selected 8 as the number of clusters to use in analyses.
Rights and permissions
About this article
Cite this article
Huang, CY., Lee, CC., Yang, HC. et al. Radiomics as prognostic factor in brain metastases treated with Gamma Knife radiosurgery. J Neurooncol 146, 439–449 (2020). https://doi.org/10.1007/s11060-019-03343-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-019-03343-4