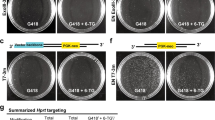
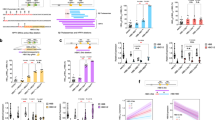
Abstract
Gene conversion is a process of transferring genetic material from one homologous sequence to another. Most reported gene conversions are meiotic although mitotic gene conversion is also described. When using CRISPR/Cas9 to target the human hemoglobin subunit beta (HBB) gene, hemoglobin subunit delta (HBD) gene footprints were observed in HBB gene. However, it is unclear whether these were the results of gene conversion or PCR-mediated sequence shuffling between highly homologous sequences. Here we provide evidence that the HBD footprints in HBB were indeed results of gene conversion. We demonstrated that the CRISPR/Cas9 facilitated unidirectional sequence transfer from the homologous gene without double-strand breaks (DSB) to the one with DSBs, and showed that the rates of HBD footprint in HBB were positively correlated to the HBB insertion and deletion rates. We further showed that when targeting HBD gene, HBB footprints could also be observed in HBD gene. The mitotic gene conversion was observed not only in immortalized HEK293T cells, but also in human primary cells. Our work reveals mitotic gene conversion as an often overlooked effect of CRISPR/Cas9-mediated genome editing.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The NGS data generated in this study were submitted to SRA with accession numbers PRJNA575526 and PRJNA590474.
References
Chen JM, Cooper DN, Chuzhanova N, Ferec C, Patrinos GP. Gene conversion: mechanisms, evolution and human disease. Nat Rev Genet. 2007;8:762–75.
Slightom JL, Blechl AE, Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980;21:627–38.
Papadakis MN, Patrinos GP. Contribution of gene conversion in the evolution of the human beta-like globin gene family. Hum Genet. 1999;104:117–25.
Zangenberg G, Huang MM, Arnheim N, Erlich H. New HLA-DPB1 alleles generated by interallelic gene conversion detected by analysis of sperm. Nat Genet. 1995;10:407–14.
Mussotter T, Bengesser K, Hogel J, Cooper DN, Kehrer-Sawatzki H. Population-specific differences in gene conversion patterns between human SUZ12 and SUZ12P are indicative of the dynamic nature of interparalog gene conversion. Hum Genet. 2014;133:383–401.
Steinberg MH, Adams JG 3rd. Hemoglobin A2: origin, evolution, and aftermath. Blood. 1991;78:2165–77.
Manchinu MF, Marongiu MF, Poddie D, Casu C, Latini V, Simbula M, et al. In vivo activation of the human delta-globin gene: the therapeutic potential in beta-thalassemic mice. Haematologica. 2014;99:76–84.
Adams JG, Morrison WT, Steinberg MH. Hemoglobin Parchman—double crossover within a single human gene. Science. 1982;218:291–3.
Petes TD. Evidence that structural variants within the human omega-globin protein may reflect genetic interactions between the omega-globin and beta-globin genes. Am J Hum Genet. 1982;34:820–3.
Petes T, Fink GR. Gene conversion between repeated genes. Nature. 1982;300:216–7.
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337:816–21.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Cho SW, Kim S, Kim JM, Kim JS. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat Biotechnol. 2013;31:230–2.
Mali P, Yang L, Esvelt KM, Aach J, Guell M, DiCarlo JE, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–6.
Jinek M, East A, Cheng A, Lin S, Ma E, Doudna J. RNA-programmed genome editing in human cells. Elife. 2013;2:e00471.
Hoban MD, Lumaquin D, Kuo CY, Romero Z, Long J, Ho M, et al. CRISPR/Cas9-mediated correction of the sickle mutation in human CD34+ cells. Mol Ther. 2016;24:1561–9.
Dever DP, Bak RO, Reinisch A, Camarena J, Washington G, Nicolas CE, et al. CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature. 2016;539:384–9.
DeWitt MA, Magis W, Bray NL, Wang T, Berman JR, Urbinati F, et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci Transl Med. 2016;8:360ra134.
Antoniani C, Meneghini V, Lattanzi A, Felix T, Romano O, Magrin E, et al. Induction of fetal hemoglobin synthesis by CRISPR/Cas9-mediated editing of the human beta-globin locus. Blood. 2018;131:1960–73.
Ye L, Wang J, Tan Y, Beyer AI, Xie F, Muench MO, et al. Genome editing using CRISPR-Cas9 to create the HPFH genotype in HSPCs: an approach for treating sickle cell disease and beta-thalassemia. Proc Natl Acad Sci USA. 2016;113:10661–5.
Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell. 2015;6:363–72.
Bothmer A, Phadke T, Barrera LA, Margulies CM, Lee CS, Buquicchio F, et al. Characterization of the interplay between DNA repair and CRISPR/Cas9-induced DNA lesions at an endogenous locus. Nat Commun. 2017;8:13905.
Bak RO, Dever DP, Reinisch A, Cruz Hernandez D, Majeti R, Porteus MH. Multiplexed genetic engineering of human hematopoietic stem and progenitor cells using CRISPR/Cas9 and AAV6. Elife. 2017;6. pii: e27873. https://doi.org/10.7554/eLife.27873.
Wang GC, Wang Y. Frequency of formation of chimeric molecules as a consequence of PCR coamplification of 16S rRNA genes from mixed bacterial genomes. Appl Environ Microbiol. 1997;63:4645–50.
Zhang K, Martiny AC, Reppas NB, Barry KW, Malek J, Chisholm SW, et al. Sequencing genomes from single cells by polymerase cloning. Nat Biotechnol. 2006;24:680–6.
Smyth RP, Schlub TE, Grimm A, Venturi V, Chopra A, Mallal S, et al. Reducing chimera formation during PCR amplification to ensure accurate genotyping. Gene. 2010;469:45–51.
Odelberg SJ, Weiss RB, Hata A, White R. Template-switching during DNA synthesis by Thermus aquaticus DNA polymerase I. Nucleic Acids Res. 1995;23:2049–57.
Szostak JW, Orr-Weaver TL, Rothstein RJ, Stahl FW. The double-strand-break repair model for recombination. Cell. 1983;33:25–35.
Lu B, Javidi-Parsijani P, Makani V, Mehraein-Ghomi F, Sarhan WM, Sun D, et al. Delivering SaCas9 mRNA by lentivirus-like bionanoparticles for transient expression and efficient genome editing. Nucleic Acids Res. 2019;47:e44.
Lyu P, Javidi-Parsijani P, Atala A, Lu B. Delivering Cas9/sgRNA ribonucleoprotein (RNP) by lentiviral capsid-based bionanoparticles for efficient ‘hit-and-run’ genome editing. Nucleic Acids Res. 2019;47:e99.
Sanada C, Kuo CJ, Colletti EJ, Soland M, Mokhtari S, Knovich MA, et al. Mesenchymal stem cells contribute to endogenous FVIII:c production. J Cell Physiol. 2013;228:1010–6.
Javidi-Parsijani P, Niu G, Davis M, Lu P, Atala A, Lu B. No evidence of genome editing activity from Natronobacterium gregoryi Argonaute (NgAgo) in human cells. Plos One. 2017;12:e0177444.
Haeussler M, Schonig K, Eckert H, Eschstruth A, Mianne J, Renaud JB, et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 2016;17:148.
Bae S, Park J, Kim JS. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics. 2014;30:1473–5.
Park J, Lim K, Kim JS, Bae S. Cas-analyzer: an online tool for assessing genome editing results using NGS data. Bioinformatics. 2017;33:286–8.
Clement K, Rees H, Canver MC, Gehrke JM, Farouni R, Hsu JY, et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat Biotechnol. 2019;37:224–6.
Cradick TJ, Fine EJ, Antico CJ, Bao G. CRISPR/Cas9 systems targeting beta-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res. 2013;41:9584–92.
Sternberg SH, Redding S, Jinek M, Greene EC, Doudna JA. DNA interrogation by the CRISPR RNA-guided endonuclease Cas9. Nature. 2014;507:62–7.
Ran FA, Cong L, Yan WX, Scott DA, Gootenberg JS, Kriz AJ, et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature. 2015;520:186–91.
Kaya H, Mikami M, Endo A, Endo M, Toki S. Highly specific targeted mutagenesis in plants using Staphylococcus aureus Cas9. Sci Rep. 2016;6:26871.
Friedland AE, Baral R, Singhal P, Loveluck K, Shen S, Sanchez M, et al. Characterization of Staphylococcus aureus Cas9: a smaller Cas9 for all-in-one adeno-associated virus delivery and paired nickase applications. Genome Biol. 2015;16:257.
Smith CE, Llorente B, Symington LS. Template switching during break-induced replication. Nature. 2007;447:102–5.
Ihry RJ, Worringer KA, Salick MR, Frias E, Ho D, Theriault K, et al. p53 inhibits CRISPR-Cas9 engineering in human pluripotent stem cells. Nat Med. 2018;24:939–46.
Deppert W, Steinmayer T, Richter W. Cooperation of SV40 large T antigen and the cellular protein p53 in maintenance of cell transformation. Oncogene. 1989;4:1103–10.
Wu Y, Liang D, Wang Y, Bai M, Tang W, Bao S, et al. Correction of a genetic disease in mouse via use of CRISPR-Cas9. Cell Stem Cell. 2013;13:659–62.
Ma H, Marti-Gutierrez N, Park SW, Wu J, Lee Y, Suzuki K, et al. Correction of a pathogenic gene mutation in human embryos. Nature. 2017;548:413–9.
Adikusuma F, Piltz S, Corbett MA, Turvey M, McColl SR, Helbig KJ, et al. Large deletions induced by Cas9 cleavage. Nature. 2018;560:E8–9.
Egli D, Zuccaro MV, Kosicki M, Church GM, Bradley A, Jasin M. Inter-homologue repair in fertilized human eggs? Nature. 2018;560:E5–7.
Haber JE. A life investigating pathways that repair broken chromosomes. Annu Rev Genet. 2016;50:1–28.
Eikenboom JC, Vink T, Briet E, Sixma JJ, Reitsma PH. Multiple substitutions in the von Willebrand factor gene that mimic the pseudogene sequence. Proc Natl Acad Sci USA. 1994;91:2221–4.
Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR-Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36:765–71.
Funding
This work is supported by the North Carolina State Grant 330054.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Javidi-Parsijani, P., Lyu, P., Makani, V. et al. CRISPR/Cas9 increases mitotic gene conversion in human cells. Gene Ther 27, 281–296 (2020). https://doi.org/10.1038/s41434-020-0126-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-020-0126-z
This article is cited by
-
CRISPR/Cas9-mediated gene knockout and interallelic gene conversion in human induced pluripotent stem cells using non-integrative bacteriophage-chimeric retrovirus-like particles
BMC Biology (2022)
-
CRISPR-Cas9 globin editing can induce megabase-scale copy-neutral losses of heterozygosity in hematopoietic cells
Nature Communications (2021)
-
Gene conversion following CRISPR/Cas9 DNA cleavage: an overlooked effect
Gene Therapy (2020)