Abstract
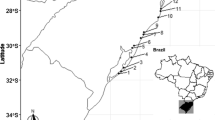
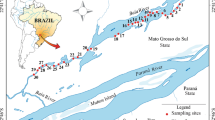
We assessed the diversity of zooplanktonic resting stages through different spatial scales in intermittent ponds along a forest–grassland transition in southern Brazil. We tested how the diversity (richness) of resting stages varied through the following spatial scales: cores (α), among sediment cores within a pond (β1), among ponds (β2), and between biomes (β3). We also assessed the diversity for the subsets of ponds within each biome. Sediment samples from 12 ponds were hydrated in the laboratory, and hatchlings were collected after 30-day incubation experiments. The estimated richness of the components β2 and β3 was higher than expected and they had the greatest contributions to total richness (47% and 24%, respectively). Within each biome, component β2 accounted for the greatest fraction of the total diversity, although their relative contributions changed between biomes (forest: 66%; grassland: 58%). The higher contribution of the among-pond spatial scale (β2) can be accounted to the environmental heterogeneity among sites. The higher contribution of the broadest spatial scales to the total diversity in the forest rather than the grassland biome suggests that vegetation type influences the spatial patterns of diversity of the zooplankton.






Similar content being viewed by others
References
Angeler, D. G. & G. García, 2005. Using emergence from soil propagule banks as indicators of ecological integrity in wetlands: advantages and limitations. Journal of the North American Benthological Society 24: 740–752.
Ávila, A. C., C. Stenert & L. Maltchik, 2011. Partitioning macroinvertebrate diversity across different spatial scales in southern Brazil coastal wetlands. Wetlands 31: 459–469.
Ávila, A. C., T. Boelter, R. M. Dos Santos, C. Stenert, N. L. Würdig, O. Rocha & L. Maltchik, 2015. The effects of different rice cultivation systems and ages on resting stages of wetland invertebrates in southern Brazil. Marine and Freshwater Research 66: 276–285.
Ávila, A. C., M. M. Pires, E. N. L. Rodrigues, J. A. C. Costi, C. Stenert & L. Maltchik, 2019. Drivers of the beta diversity of spider assemblages in southern Brazilian temporary wetlands. Ecological Entomology. https://doi.org/10.1111/een.12816.
Belton, W., 1994. Aves do Rio Grande do Sul: distribuição e biologia. Editora Unisinos, São Leopoldo.
Bertuzzi, T., M. M. Pires & L. Maltchik, 2019. Drivers of the beta diversity of aquatic plant communities along a latitudinal gradient in southern Brazilian coastal ponds. Journal of Vegetation Science 30: 281–290.
Boven, L., B. Vanschoenwinkel, E. R. De Roeck, A. Hulsmans & L. Brendonck, 2008. Diversity and distribution of large branchiopods in Kiskunság (Hungary) in relation to local habitat and spatial factors: implications for their conservation. Marine and Freshwater Research 59: 940–950.
Brock, M. A., D. L. Nielsen, R. J. Shiel, J. D. Green & J. D. Langley, 2003. Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshwater Biology 48: 1207–1218.
Chaparro, G., Z. Horváth, I. O’Farrell, R. Ptacnik & T. Hein, 2018. Plankton metacommunities in floodplain wetlands under contrasting hydrological conditions. Freshwater Biology 63: 380–391.
Chase, J. M. & M. A. Leibold, 2002. Spatial scale dictates the productivity–biodiversity relationship. Nature 416: 427−430.
Chesson, P., 2000. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics 31: 343–366.
Cottenie, K. & L. De Meester, 2004. Metacommunity structure: synergy of biotic interactions as selective agents and dispersal as fuel. Ecology 85: 114–119.
Cottenie, K., E. Michels, N. Nuytten & L. De Meester, 2003. Zooplankton metacommunity structure: regional vs. local processes in highly interconnected ponds. Ecology 84: 991–1000.
Crist, T. O., J. A. Veech, J. C. Gering & K. S. Summerville, 2003. Partitioning species diversity across landscapes and regions: a hierarchical analysis of α, β, and γ diversity. The American Naturalist 162: 734–743.
De Meester, L., S. Declerck, R. Stoks, G. Louette, F. Van De Meutter, T. De Bie, E. Michels & L. Brendonck, 2005. Ponds and pools as model systems in conservation biology, ecology and evolutionary biology. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 715–725.
De Stasio, J. B. T., 1990. The role of dormancy and emergence patterns in the dynamics of a freshwater zooplankton community. Limnology and Oceanography 35: 1079–1090.
Declerck, S. A., J. S. Coronel, P. Legendre & L. Brendonck, 2011. Scale dependency of processes structuring metacommunities of cladocerans in temporary pools of High-Andes wetlands. Ecography 34: 296–305.
Dittrich, J., J. D. Dias, C. C. Bonecker, F. A. Lansac-Tôha & A. A. Padial, 2016. Importance of temporal variability at different spatial scales for diversity of floodplain aquatic communities. Freshwater Biology 61: 316–327.
Dumbrell, A. J., E. J. Clark, G. A. Frost, T. E. Randell, J. W. Pitchford & J. K. Hill, 2008. Changes in species diversity following habitat disturbance are dependent on spatial scale: theoretical and empirical evidence. Journal of Applied Ecology 45: 1531–1539.
Elmoor-Loureiro, L. M. A., 1997. In Coelho, R. (ed.), Manual de identificação de cladóceros límnicos do Brazil. Editora Universa, Brasília: 1–155.
Elmoor-Loureiro, L. M. A., 2000. Cladóceros do Brasil: famílias Chydoridae e Eurycercidae [available on internet at https://cladocera.wordpress.com]. Accessed 1 Apr 2018.
Fernández, A. I., O. Viedma, S. Sánchez-Carrillo, M. Alvarez-Cobelas & D. G. Angeler, 2009. Local and landscape effects on temporary pond zooplankton egg banks: conservation implications. Biodiversity and Conservation 18: 2373–2386.
Fleishman, E., C. J. Betrus & R. B. Blair, 2003. Effects of spatial scale and taxonomic group on partitioning of butterfly and bird diversity in the Great Basin, USA. Landscape Ecology 18: 675–685.
Florencio, M., C. Díaz-Paniagua, C. Gómez-Rodríguez & L. Serrano, 2014. Biodiversity patterns in a macroinvertebrate community of a temporary pond network. Insect Conservation and Diversity 7: 4–21.
Freiry, R. F., F. M. Esquinatti, C. Stenert, A. Arenzon, D. L. Nielsen & L. Maltchik, 2016. Effects of spatial scale and habitat on the diversity of diapausing wetland invertebrates. Aquatic Biology 25: 173–181.
Freiry, R. F., A. Gouvêa, J. Becker, F. A. Lansac-Tôha, F. M. Lansac-Tôha, M. M. Pires, C. Stenert & L. Maltchik, 2019. Community structure and concordance patterns among zooplankton life stages in subtropical temporary ponds. Aquatic Ecology. https://doi.org/10.1007/s10452-019-09740-1.
Gazulha, V., 2012. Zooplâncton límnico: manual ilustrado. Technical Books Editora, Rio de Janeiro.
Gerhard, M., C. Iglesias, J. M. Clemente, G. Goyenola, M. Meerhoff, J. P. Pacheco, F. Teixeira-de Mello & N. Mazzeo, 2017. What can resting egg banks tell about cladoceran diversity in a shallow subtropical lake? Hydrobiologia 798: 75–86.
Gering, J. C., T. O. Crist & J. A. Veech, 2003. Additive partitioning of species diversity across multiple spatial scales: implications for regional conservation of biodiversity. Conservation Biology 17: 488–499.
Giller, P. S., A. G. Hildrew & D. G. Raffaelli, 1994. Aquatic Ecology: Scale, Pattern and Process. Blackwell Science, Oxford.
Godfray, H. C. J. & J. H. Lawton, 2001. Scale and species numbers. Trends in Ecology and Evolution 16: 400–404.
Gotelli, N. J. & R. K. Colwell, 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391.
Green, A. J. & J. Figuerola, 2005. Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Diversity and Distributions 11: 149–156.
Green, A. J., K. M. Jenkins, D. Bell, P. J. Morris & R. T. Kingsford, 2008. The potential role of waterbirds in dispersing invertebrates and plants in arid Australia. Freshwater Biology 53: 380–392.
Gyllström, M. & L. A. Hansson, 2004. Dormancy in freshwater zooplankton: induction, termination and the importance of benthic–pelagic coupling. Aquatic Sciences 66: 274–295.
Hairston, J. N. G., 1996. Zooplankton egg banks as biotic reservoirs in changing environments. Limnology and Oceanography 41: 1087–1092.
Hairston, J. N. G. & C. M. Kearns, 2002. Temporal dispersal: ecological and evolutionary aspects of zooplankton egg banks and the role of sediment mixing. Integrative and Comparative Biology 42: 481–491.
Hairston, J. N. G., R. A. Van Brunt, C. M. Kearns & D. R. Engstrom, 1995. Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76: 1706–1711.
Havel, J. E. & J. B. Shurin, 2004. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnology and Oceanography 49: 1229–1238.
Havel, J. E., W. R. Mabee & J. R. Jones, 1995. Invasion of the exotic cladoceran Daphnia lumholtzi into North American reservoirs. Canadian Journal of Fisheries and Aquatic Sciences 52: 151–160.
Heino, J. & B. L. Peckarsky, 2014. Integrating behavioral, population and large-scale approaches for understanding stream insect communities. Current Opinion in Insect Science 2: 7–13.
Heino, J., P. Louhi & T. Muotka, 2004. Identifying the scales of variability in stream macroinvertebrate abundance, functional composition and assemblage structure. Freshwater Biology 49: 1230–1239.
Heino, J., A. S. Melo, L. M. Bini, F. Altermatt, S. A. Al-Shami, D. G. Angeler, N. Bonada, C. Brand, M. Callisto, K. Cottenie, O. Dangles, D. Dudgeon, A. Encalada, E. Göthe, M. Grönroos, N. Hamada, D. Jacobsen, V. L. Landeiro, R. Ligeiro, R. T. Martins, M. L. Miserendino, C. S. M. Rawi, M. E. Rodrigues, F. O. Roque, L. Sandin, D. Schmera, L. F. Sgarbi, J. P. Simaika, T. Siqueira, R. M. Thompson & C. R. Townsend, 2015a. A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecology and Evolution 5: 1235–1248.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. Valanko & L. M. Bini, 2015b. Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biology 60: 845–869.
Henriques-Silva, R., B. Pinel-Alloul & P. R. Peres-Neto, 2016. Climate, history and life-history strategies interact in explaining differential macroecological patterns in freshwater zooplankton. Global Ecology and Biogeography 25: 1454–1465.
Hepp, L. U. & A. S. Melo, 2013. Dissimilarity of stream insect assemblages: effects of multiple scales and spatial distances. Hydrobiologia 703: 239–246.
Hessen, D. O., T. C. Jensen & B. Walseng, 2019. Zooplankton diversity and dispersal by birds; insights from different geographical scales. Frontiers in Ecology and Evolution 7: e74.
Horváth, Z., C. F. Vad & R. Ptacnik, 2016. Wind dispersal results in a gradient of dispersal limitation and environmental match among discrete aquatic habitats. Ecography 39: 726–732.
Instituto Brasileiro de Geografia e Estatística, IBGE, 2004. Mapa de Biomas do Brasil. IBGE, Rio de Janeiro.
Ketmaier, V., F. Marrone, G. Alfonso, K. Paulus, A. Wiemann, R. Tiedemann & G. Mura, 2012. Mitochondrial DNA regionalism and historical demography in the extant populations of Chirocephalus kerkyrensis (Branchiopoda: Anostraca). PLoS ONE 7: e30082.
Knauth, D. K., M. M. Pires, C. Stenert & L. Maltchik, 2019. Disentangling the role of niche-based and spatial processes on anuran beta diversity in temporary ponds along a forest–grassland transition. Aquatic Sciences 81: e63.
Koste, W., 1978. Rotatoria. Gebrüder Bosntraeget, Stuttgart.
Lande, R., 1996. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 76: 5–13.
Leão, T. C., C. R. Fonseca, C. A. Peres & M. Tabarelli, 2014. Predicting extinction risk of Brazilian Atlantic Forest angiosperms. Conservation Biology 28: 1349–1359.
Legendre, P., 2014. Interpreting the replacement and richness difference components of beta diversity. Global Ecology and Biogeography 23: 1324–1334.
Legendre, P. & M. J. Anderson, 1999. Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69: 1–24.
Legendre, P. & L. F. J. Legendre, 2012. Numerical Ecology. Elsevier, Amsterdam.
Leibold, M. A. & J. M. Chase, 2018. Processes in metacommunities. In Leibold, M. A. & J. M. Chase (eds), Metacommunity Ecology. Princeton University Press, Princeton: 49–89.
Levin, S. A., 1992. The problem of pattern and scale in ecology: the Robert H. MacArthur Award lecture. Ecology 73: 1943–1967.
Ligeiro, R., A. S. Melo & M. Callisto, 2010. Spatial scale and the diversity of macroinvertebrates in a Neotropical catchment. Freshwater Biology 55: 424–435.
Maltchik, L., 2003. Inventory of wetlands of Rio Grande do Sul (Brazil). Pesquisas Bot 53: 89–100.
Maluf, J. R. T., 2000. Nova classificação climática do Estado do Rio Grande do Sul. Revista Brasileira de Agrometeorologia 8: 141–150.
Mazei, Y., 2008. Biodiversity patterns in protozoan communities: linking processes and scales. Protistology 5: 268–280.
Melo, T. X. & E. S. Medeiros, 2013. Spatial distribution of zooplankton diversity across temporary pools in a semiarid intermittent river. International Journal of Biodiversity 2013: e946361.
Menge, B. A. & A. M. Olson, 1990. Role of scale and environmental factors in regulation of community structure. Trends in Ecology and Evolution 5: 52–57.
Mikulski, A. & J. Pijanowska, 2009. Maternal experience can enhance production of resting eggs in Daphnia exposed to the risk of fish predation. Fundamental and Applied Limnology/Archiv für Hydrobiologie 174: 301–305.
Montero-Pau, J., A. Gómez & M. Serra, 2018. Founder effects drive the genetic structure of passively dispersed aquatic invertebrates. PeerJ 6: e6094.
Morais-Junior, C. S., M. Melo-Júnior, T. Gonçalves-Souza & R. M. de Lyra-Neves, 2019. Zoochory of zooplankton: seasonality and bird morphological diversity can influence metacommunity dynamics of temporary ponds. Journal of Plankton Research 41: 465–477.
Moreno, E., C. Pérez-Martínez & J. M. Conde-Porcuna, 2019. Dispersal of rotifers and cladocerans by waterbirds: seasonal changes and hatching success. Hydrobiologia 834: 145–162.
Ng, I. S., C. M. Carr & K. Cottenie, 2009. Hierarchical zooplankton metacommunities: distinguishing between high and limiting dispersal mechanisms. Hydrobiologia 619: 133–143.
Oksanen, J., F. G. Blanchet, M. Friendly, R. Kindt, P. Legendre, D. McGlinn, P. R. Minchin, R. B. O’Hara, G. L, Simpson, P. Solymos, M. H. H. Stevens, E. Szoecs & H. Wagner, 2018. Package ‘vegan’ Community Ecology Package. [available on internet at https://cran.r-512project.org/package=vegan]. Accessed 1 Nov 2018.
Palazzo, F., C. C. Bonecker & A. P. C. Fernandes, 2008. Resting cladoceran eggs and their contribution to zooplankton diversity in a lagoon of the Upper Paraná River Floodplain. Lakes and Reservoirs: Research and Management 13: 207–214.
Panarelli, E. A., S. M. C. Casanova & R. Henry, 2008. The role of resting eggs in the recovery of zooplankton community in a marginal lake of the Paranapanema River (São Paulo, Brazil), after a long drought period. Acta Limnologica Brasiliensia 20: 73–88.
Parekh, P. A., M. J. Paetkau & L. A. Gosselin, 2014. Historical frequency of wind dispersal events and role of topography in the dispersal of anostracan cysts in a semi-arid environment. Hydrobiologia 740: 51–59.
Pedruski, M. T. & S. E. Arnott, 2011. The effects of habitat connectivity and regional heterogeneity on artificial pond metacommunities. Oecologia 166: 221–228.
Perbiche-Neves, G., V. S. Saito, N. R. Simões, J. R. Debastiani-Júnior, D. A. O. Naliato & M. G. Nogueira, 2019. Distinct responses of Copepoda and Cladocera diversity to climatic, environmental, and geographic filters in the La Plata River Basin. Hydrobiologia 826: 113–127.
Peres-Neto, P. R., P. Legendre, S. Dray & D. Borcard, 2006. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87: 2614–2625.
Piercey, D. W. & E. J. Maly, 2000. Factors influencing the induction of diapausing egg production in the calanoid copepod Diaptomus leptopus. Aquatic Ecology 34: 9–17.
Pinceel, T., L. Brendonck & B. Vanschoenwinkel, 2016. Propagule size and shape may promote local wind dispersal in freshwater zooplankton – a wind tunnel experiment. Limnology and Oceanography 61: 122–131.
Pires, M. M., C. B. Kotzian, M. R. Spies & V. A. Baptista, 2016. Comparative assessment of aquatic macroinvertebrate diversity in irrigated rice fields and wetlands through different spatial scales: an additive partitioning approach. Marine and Freshwater Research 67: 368–379.
Pires, M. M., C. Stenert & L. Maltchik, 2018. Drivers of beta diversity of Odonata along a forest–grassland transition in southern Brazilian coastal ponds. Freshwater Science 37: 357–366.
Portinho, J. L., D. L. Nielsen, N. Ning, W. Paul & M. Nogueira, 2017. Spatial variability of aquatic plant and microfaunal seed and egg bank communities within a forested floodplain system of a temperate Australian river. Aquatic Sciences 79: 515–527.
R Core Team, 2018. R: A Language and Environment for Statistical Computing.
Rosário, L. D., 1996. As aves em Santa Catarina: distribuição geográfica e meio ambiente. Fatma, Florianópolis.
Santangelo, J. M., F. D. A. Esteves, M. Manca & R. L. Bozelli, 2011. Abundance, composition and spatial variation in the egg bank of a tropical zooplankton community. Studies on Neotropical Fauna and Environment 46: 225–232.
Santangelo, J. M., P. M. Lopes, M. O. Nascimento, A. P. C. Fernandes, S. Bartole, M. P. Figueiredo-Barros, J. J. F. Leal, F. A. Esteves, V. F. Farjalla, C. C. Bonecker & R. L. Bozelli, 2015. Community structure of resting egg banks and concordance patterns between dormant and active zooplankters in tropical lakes. Hydrobiologia 758: 183–195.
Segers, H., 2004. Rotifera Monogononta. In Yule, C. M. & H. S. Yong (eds), Freshwater Invertebrates of the Malaysia Region. Academy of Sciences and Monash University, Malaysia, Kuala Lumpur: 112–116.
Shurin, J. B., J. E. Havel, M. A. Leibold & B. Pinel-Alloul, 2000. Local and regional zooplankton species richness: a scale-independent test for saturation. Ecology 81: 3062–3073.
Shurin, J. B., K. Cottenie & H. Hillebrand, 2009. Spatial autocorrelation and dispersal limitation in freshwater organisms. Oecologia 159: 151–159.
Silva, G. G., A. J. Green, V. Weber, P. Hoffmann, Á. Lovas-Kiss, C. Stenert & L. Maltchik, 2018. Whole angiosperms Wolffia columbiana disperse by gut passage through wildfowl in South America. Biology Letters 14: 20180703.
Silva, G. G., V. Weber, A. J. Green, P. Hoffmann, V. S. Silva, M. Volcan, L. E. K. Lanés, C. Stenert, M. Reichard & L. Maltchik, 2019. Killifish eggs can disperse via gut passage through waterfowl. Ecology 100: e02774.
Simões, N. R., J. D. Dias, C. M. Leal, L. D. S. M. Braghin, F. A. Lansac-Tôha & C. C. Bonecker, 2013. Floods control the influence of environmental gradients on the diversity of zooplankton communities in a Neotropical floodplain. Aquatic Sciences 75: 607–617.
Slusarczyk, M., A. Ochocka & D. Cichocka, 2012. The prevalence of diapause response to risk of size-selective predation in small-and large-bodied prey species. Aquatic Ecology 46: e1.
Stenert, C., R. C. Bacca, A. C. Ávila, L. Maltchik & O. Rocha, 2010. Do hydrologic regimes used in rice fields compromise the viability of resting stages of aquatic invertebrates? Wetlands 30: 989–996.
Stenert, C., R. Wüsth, M. M. Pires, R. F. Freiry, D. Nielsen & L. Maltchik, 2017. Composition of cladoceran dormant stages in intermittent ponds with different hydroperiod lengths. Ecological Research 32: 921–930.
Stoch, F., M. Korn, S. Turki, L. Naselli-Flores & F. Marrone, 2016. The role of spatial environmental factors as determinants of large branchiopod distribution in Tunisian temporary ponds. Hydrobiologia 782: 37–51.
Stross, R. G. & J. C. Hill, 1965. Diapause induction in Daphnia requires two stimuli. Science 150: 1462–1464.
Tomazelli, L., 1993. O regime dos ventos e a taxa de migração das dunas eólicas costeiras do Rio Grande do Sul, Brasil. Pesquisas em Geociências 20: 18–26.
Van Leeuwen, C. H., G. Van der Velde, J. M. van Groenendael & M. Klaassen, 2012. Gut travellers: internal dispersal of aquatic organisms by waterfowl. Journal of Biogeography 39: 2031–2040.
Vanschoenwinkel, B., S. Gielen, M. Seaman & L. Brendonck, 2008. Any way the wind blows-frequent wind dispersal drives species sorting in ephemeral aquatic communities. Oikos 117: 125–134.
Vanschoenwinkel, B., S. Gielen, M. Seaman & L. Brendonck, 2009. Wind mediated dispersal of freshwater invertebrates in a rock pool metacommunity: differences in dispersal capacities and modes. Hydrobiologia 635: 363–372.
Vargas, A. L., J. M. Santangelo & R. L. Bozelli, 2019. Recovery from drought: viability and hatching patterns of hydrated and desiccated zooplankton resting eggs. International Review of Hydrobiology 104: 26–33.
Veech, J. A. & T. O. Crist, 2007. Habitat and climate heterogeneity maintain beta-diversity of birds among landscapes within ecoregions. Global Ecology and Biogeography 16: 650–656.
Veech, J. A., K. S. Summerville, T. O. Crist & J. C. Gering, 2002. The additive partitioning of species diversity: recent revival of an old idea. Oikos 99: 3–9.
Vendramin, D., C. S. Klagenberg, M. R. Provensi, C. Stenert, M. M. Pires, E. S. F. Medeiros, M. Reichard & L. Maltchik, 2020. Effects of the presence of annual killifish on the assemblage structure of resting stages of aquatic invertebrates in temporary ponds. Limnetica 39: 1−16.
Ventura, M., A. Petrusek, A. Miró, E. Hamrová, D. Buñay, L. De Meester & J. Mergeay, 2014. Local and regional founder effects in lake zooplankton persist after thousands of years despite high dispersal potential. Molecular Ecology 23: 1014–1027.
Viana, D. S., L. Santamaría, T. C. Michot & J. Figuerola, 2013. Migratory strategies of waterbirds shape the continental-scale dispersal of aquatic organisms. Ecography 36: 430–438.
Viana, D. S., J. Figuerola, K. Schwenk, M. Manca, A. Hobæk, M. Mjelde, C. D. Preston, J. R. Gornall, J. M. Croft, R. A. King, A. J. Green & L. Santamaria, 2016. Assembly mechanisms determining high species turnover in aquatic communities over regional and continental scales. Ecography 39: 281–288.
Villwock, J. A. & L. J. Tomazelli, 2006. Planície Costeira do Rio Grande do Sul: gênese e paisagem atual. In Becker, F. G., R. A. Ramos & L. A. Moura (eds), Biodiversidade. Regiões da Lagoa do Casamento e dos Butiazais de Tapes, Planície costeira do Rio Grande do Sul. Ministério do Meio Ambiente, Brasília: 20–33.
Walsh, M. R., 2013. The link between environmental variation and evolutionary shifts in dormancy in zooplankton. Integrative and Comparative Biology 53: 713–722.
Waters, J. M., C. I. Fraser & G. M. Hewitt, 2013. Founder takes all: density-dependent processes structure biodiversity. Trends in Ecology and Evolution 28: 78–85.
Whittaker, R. H., 1960. Vegetation of the Siskiyou Mountains, Oregon and California. Ecological Monographs 30: 279–338.
Whittaker, R. H., 1972. Evolution and measurement of species diversity. Taxon 21: 213–251.
Wiens, J. A., 1989. Spatial scaling in ecology. Functional Ecology 3: 385–397.
Williams, D., 1998. The role of dormancy in the evolution and structure of temporary water invertebrate communities. Archiv für Hydrobiologie 52: 109–124.
Williams, P., M. Whitfield, J. Biggs, S. Bray, G. Fox, P. Nicolet & D. Sear, 2003. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biological Conservation 115: 329–341.
Wu, J. & O. L. Loucks, 1995. From balance of nature to hierarchical patch dynamics: a paradigm shift in ecology. The Quarterly Review of Biology 70: 439–466.
Yampolsky, L. Y., 1992. Genetic variation in the sexual reproduction rate within a population of a cyclic parthenogen, Daphnia magna. Evolution 46: 833–837.
Zadereev, E. & T. Lopatina, 2007. The induction of diapause in Moina by species-specific chemical cues. Aquatic Ecology 41: 255–261.
Acknowledgements
We thank Dr. Francisco Diogo Rocha Sousa (Federal University of Jataí) for collaboration in the identification of the zooplankton. We are grateful to Andressa Gouvea, and Jennifer Becker for their help in laboratory work. This study was funded by the Brazilian National Council for Scientific and Technological Development (CNPq: Grant Number 474892/2013-1). LM, CS, CCB, and FALT hold CNPq Research Productivity Grants. RFF received a PhD Scholarship from CAPES (Coordination for the Improvement of Higher Level Personnel) and MMP received a Postdoctoral Fellowship from CNPq (Grant Number 151152/2018-7) at UNISINOS Biology Graduate Program.
Author information
Authors and Affiliations
Contributions
RFF collected the material, conducted the laboratory experiments, specimen identification and participated in the drafting of the manuscript. VW collaborated with the laboratory experiments. CCB and FALT participated in specimen identification and drafting of the manuscript. MMP performed the statistical analyses and participated in the drafting of the manuscript. LM and CS coordinated several aspects of the study, including revision of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
We declare that the data collection complied with the current Brazilian environmental laws (SISBIO 36365-2).
Additional information
Handling editor: Dani Boix
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10750_2020_4187_MOESM1_ESM.xlsx
Supplementary material 1 (XLSX 26 kb). Table S2 Composition and number of individuals of dormant stages of zooplankton recorded in the 12 ponds studied in the southern Brazilian Coastal Plain
Rights and permissions
About this article
Cite this article
Freiry, R.F., Weber, V., Bonecker, C.C. et al. Additive partitioning of the diversity of the dormant zooplankton communities in intermittent ponds along a forest–grassland transition. Hydrobiologia 847, 1327–1342 (2020). https://doi.org/10.1007/s10750-020-04187-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-020-04187-0