Abstract
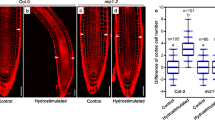
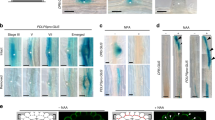
Root branching is influenced by the soil environment and exhibits a high level of plasticity. We report that the radial positioning of emerging lateral roots is influenced by their hydrological environment during early developmental stages. New lateral root primordia have both a high degree of flexibility in terms of initiation and development angle towards the available water. Our observations reveal how the external hydrological environment regulates lateral root morphogenesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Morris, E. C. et al. Shaping 3D root system architecture. Curr. Biol. 27, R919–R930 (2017).
Drew, M. C. Comparison of the effects of a localized supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. N. Phytol. 75, 479–490 (1975).
Orman-Ligeza, B. et al. The xerobranching response represses lateral root formation when roots are not in contact with water. Curr. Biol. 28, 3165–3173 (2018).
Bao, Y. et al. Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc. Natl Acad. Sci. USA 111, 9319–9324 (2014).
Orosa-Puente, B. et al. Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 362, 1407–1410 (2018).
von Guttenberg, H. in Handbuch der Pflanzenanatomie (ed. Linsbauer, K.) Band 8 (Gebrüder Bornträger, 1940).
Casero, P. J., Casimiro, I. & Lloret, P. G. Lateral root initiation by asymmetrical transverse divisions of pericycle cells in four plant species: Raphanus sativus, Helianthus annuus, Zea mays, and Daucus carota. Protoplasma 188, 49–58 (1995).
Laskowski, M. J., Williams, M. E., Nusbaum, H. C. & Sussex, I. M. Formation of lateral root meristems is a two-stage process. Development 121, 3303–3310 (1995).
Casimiro, I. et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13, 843–852 (2001).
Dubrovsky, J. G., Rost, T. L., Colón-Carmona, A. & Doerner, P. Early primordium morphogenesis during lateral root initiation in Arabidopsis thaliana. Planta 214, 30–36 (2001).
Lucas, M. et al. Lateral root morphogenesis is dependent on the mechanical properties of the overlaying tissues. Proc. Natl Acad. Sci. USA 110, 5229–5234 (2013).
von Wangenheim, D. et al. Rules and self-organizing properties of post-embryonic plant organ cell division patterns. Curr. Biol. 26, 439–449 (2016).
Casimiro, I. et al. Dissecting Arabidopsis lateral root development. Trends Plant Sci. 8, 165–171 (2003).
Goh, T., Joi, S., Mimura, T. & Fukaki, H. The establishment of asymmetry in Arabidopsis lateral root founder cells is regulated by LBD16/ASL18 and related LBD/ASL proteins. Development 139, 883–893 (2012).
Stelzer, E. H. K. Light-sheet fluorescence microscopy for quantitative biology. Nat. Methods 12, 23–26 (2015).
von Wangenheim, D., Hauschild, R. & Friml, J. Light sheet fluorescence microscopy of plant roots growing on the surface of a gel. J. Vis. Exp. 2017, e55044 (2017).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Preibisch, S., Saalfeld, S., Schindelin, J. & Tomancak, P. Software for bead-based registration of selective plane illumination microscopy Data. Nat. Methods 7, 418–419 (2010).
Preibisch, S. et al. Efficient Bayesian-based multiview deconvolution. Nat. Methods 11, 645–648 (2014).
Acknowledgements
This work was supported by awards from the Biotechnology and Biological Sciences Research Council (grant nos. BB/M012212, BB/G023972/1, BB/R013748/1, BB/L026848/1, BB/M018431/1, BB/PO16855/1 and BB/M001806/1), the European Research Council FUTUREROOTS Advanced Investigator (grant no. 294729) and the Leverhulme Trust (grant no. RPG-2016-409). E.H.K.S. is funded by the Deutsche Forschungsgemeinschaft (CEF-MC I/II, DFG Exc 115). Research at the Maizel Lab is supported by the DFG FOR2581, the Land Baden-Württemberg, the Chica und Heinz Schaller Stiftung, the CellNetworks cluster of excellence and the Boehringer Ingelheim Foundation.
Author information
Authors and Affiliations
Contributions
D.v.W., J. Banda, A.B., A.M., E.H.K.S. and M.B. designed the experiments. D.v.W. and J. Banda performed the experiments, and D.v.W., J. Banda, A.S., J. Boland, A.B., A.M., E.H.K.S. and M.B. analysed the data. D.v.W., J. Banda and M.B. wrote the manuscript with contributions from all the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4 with legends.
Supplementary Video 1
Lateral root angle dataset.
Supplementary Video 2
3D view of Fig. 2d–g.
Supplementary Data 1
Statistical Source Data.
Rights and permissions
About this article
Cite this article
von Wangenheim, D., Banda, J., Schmitz, A. et al. Early developmental plasticity of lateral roots in response to asymmetric water availability. Nat. Plants 6, 73–77 (2020). https://doi.org/10.1038/s41477-019-0580-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-019-0580-z
This article is cited by
-
The growth of capillary networks by branching for maximum fluid access
Scientific Reports (2023)
-
Plant multiscale networks: charting plant connectivity by multi-level analysis and imaging techniques
Science China Life Sciences (2021)
-
Root architecture and hydraulics converge for acclimation to changing water availability
Nature Plants (2020)