Abstract
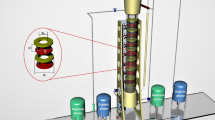
Batch and continuous experiments were carried out in a Scheibel extraction column to separate La(III) from Ce(III). Central composite design was used to evaluate the influence of pH, extractant concentration in the batch experiments, and the influence of rotor speed, phase flow rates in the continuous experiments. At optimum conditions (pH 3.5, D2EHPA extractant concentration 0.05 M, rotor speed 128 rpm, dispersed phase flow rate 32 L/h and continuous phase flow rate 18 L/h), high extraction efficiency and separation factor equal to 88.12% and 4.89, respectively, for cerium separation from lanthanum were reasonably well predicted by the model. At higher rotor speed, La(III) and Ce(III) ions move faster from aqueous to the organic phase, which retards the higher interaction between ions and D2EHPA extractant. The results showed that this extraction column could be a potential candidate for the extraction and separation of La(III) and Ce(III) ions or other industrial wastewater.
Similar content being viewed by others
References
D. A. Atwood, The rare earth elements: fundamentals and application, Wiley, New York (2016).
N. Krishnamurthy and C. K. Gupta, Extractive metallurgy of rare earths, CRC Press, New York (2016).
J. Zhang, B. Zhao and B. Schreiner, Separation Hydrometallurgy of Rare Earth Elements, Springer, New York (2016).
D. L. Ramasamy, S. Khan, E. Repo and Sillanpää M, Chem. Eng. J., 322, 56 (2017).
S. Ftekhar, V. Srivastava, S. B. Hammouda and M. Sillanpää, Carbohydr. Polym., 194, 274 (2018).
W. Zhang and R. Q. Honaker, Int. J. Coal Geology, 195, 189 (2018).
X. Yin, Y. Wang, X. Bai, Y. Wang, L. Chen, C. Xiao, J. Diwu, S. Du, Z. Chai, T. E. Albrecht-Schmitt and S. Wang, Nature Comm., 8, 14438 (2017).
F. Zhang, W. Wu, X. Bian and W. Zeng, Hydrometallurgy, 149, 238 (2014).
R. Habibpour, M. Dargahi, E. Kashi and M. Bagherpour, Metall. Res. Technol., 115, 207 (2018).
P. Ramakul, N. Leepipatpiboon, C. Yamoum, U. Thubsuang, S. Bunnak and U. Pancharoen, Korean J. Chem. Eng., 26, 765 (2009).
F. Jiang, S. Yin, C. Srinivasakannan, S. Li and J. Peng, Chem. Eng. J., 334, 2208 (2018).
K. H. Ryu, C. Lee, G. G. Lee, S. Jo and S. W. Sung, Korean J. Chem. Eng., 30, 1946 (2013).
E. Kashi, R. Habibpour, H. Gorzin and A. Maleki, J. Rare Earths, 36, 317 (2018).
Abhilash, S. Sinha, M. K. Sinha and B. D. Pandey, Inter. J. Miner. Process, 127, 70 (2014).
S. K. Sahu and S. Mishra, Sep. Sci. Technol., 51, 447 (2016).
R. Banda, H. S. Jeon and M. S. Lee, Metal. Mater. Tran. B, 45, 2009 (2014).
E. Padhan and K. Sarangi, Hydrometallurgy, 167, 134 (2017).
R. Prakorn, P. Weerawat and P. Ura, Korean J. Chem. Eng., 23, 85 (2006).
R. Prakorn and P. Ura, Korean J. Chem. Eng., 20, 724 (2003).
S. Roy, S. Basu, M. Anitha and D. K. Singh, Korean J. Chem. Eng., 34, 1740 (2017).
N. Song, X. Zhao, Q. Jia, W. Zhou and W. Liao, Korean J. Chem. Eng., 27, 1258 (2010).
F. Xie, T. A. Zhang, D. Dreisinger and F. Doyle, Miner. Eng., 56, 10 (2014).
G. Angelov and C. Gourdon, Korean J. Chem. Eng., 32, 37 (2015).
P. Amani, M. Amani and R. Hasanvandian, Korean J. Chem. Eng., 34, 1456 (2017).
Y. K. Lee, D. P. Ju and C. Kim, Korean J. Chem. Eng., 8, 80 (1991).
S. C. Lee and G. H. Hyun, Korean J. Chem. Eng., 19, 827 (2002).
T. C. Lo, Commercial liquid-liquid extraction equipment, in Handbook of separation techniques for chemical engineers, P. A. Schweitzer, McGraw-Hill, New York (1979).
J. D. Thornton, Science and Practice of Liquid-Liquid Extraction, Oxford University Press, Oxford (1992).
H. J. Bart, Reactive Extraction, Springer, New York (2001).
J. Rydberg, M. Cox, C. Musikas and G. R. Choppin, Solvent Extraction: Principles and Practice, Marcel Dekker, New York (2004).
G. M. Ritcey and A. W. Ashbrook, Solvent extraction: principles and applications to process metallurgy, Elsevier, New York (1984).
M. Asadollahzadeh, M. Torab-Mostaedi, S. Shahhosseini and A. Ghaemi, Chem. Eng. Process, 100, 65 (2016).
R. Torkaman, M. Asadollahzadeh and M. Torab-Mostaedi, Chem. Eng. Process, 111, 7 (2017).
M. Asadollahzadeh, M. Torab-Mostaedi and R. Torkaman, Chem. Eng. Res. Des., 127, 146 (2017).
M. Torab-Mostaedi, H. Jalilvand and M. Outokesh, Chem. Ind. Chem. Eng. Q., 17, 333 (2011).
M. Asadollahzadeh, M. Torab-Mostaedi and R. Torkaman, Chem. Eng. Process, 109, 97 (2016).
M. Asadollahzadeh, M. Torab-Mostaedi, S. Shahhosseini and A. Ghaemi, Chem. Eng. Res. Des., 105, 177 (2016).
M. Asadollahzadeh, M. Torab-Mostaedi, S. Shahhosseini, A. Ghaemi and R. Torkaman, Sep. Purif. Technol., 158, 275 (2016).
Sh. Houshyar, M. Torab-Mostaedi and S. H. Mousavi, J. Chem. Pet. Eng., 51, 105 (2017).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Asadollahzadeh, M., Torkaman, R. & Torab-Mostaedi, M. Study on the feasibility of using a pilot plant Scheibel extraction column for the extraction and separation of lanthanum and cerium from aqueous solution. Korean J. Chem. Eng. 37, 322–331 (2020). https://doi.org/10.1007/s11814-019-0443-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-019-0443-3