Abstract
Summary
By Bayesian random effects network meta-analysis stratified by prevalent vertebral fracture (PVF), we conclude that different effective drugs should be used to prevent fragility fractures according to postmenopausal women with or without PVF and that there are two drugs (i.e., parathyroid hormone (1-84) and abaloparatide) less tolerated than placebo.
Introduction
No studies have compared various osteoporosis drugs in postmenopausal women (PMW) either with or without prevalent vertebral fracture (PVF). We aimed to compare them in the two different subgroups.
Methods
We searched different databases to select relevant studies. We performed Bayesian random effects network meta-analysis to synthesize hazard ratio (HR) and 95% confidence interval (CI) for clinical fracture stratified by PVF and to synthesize risk ratio (RR) for tolerability and vertebral fracture.
Results
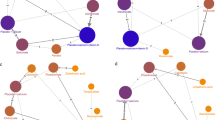
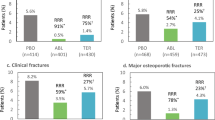
We included 33 trials involving 79,144 PMW. In the PVF ≥ 50% subgroup, teriparatide (HR 0.39, 95% CI 0.28–0.57), romosozumab (HR 0.49, 95% CI 0.29–0.75), risedronate (HR 0.62, 95% CI 0.50–0.79), zoledronate (HR 0.67, 95% CI 0.47–0.96), and alendronate (HR 0.69, 95% CI 0.47–0.97) reduced clinical fracture risk. In the other subgroup, abaloparatide (HR 0.56, 95% CI 0.33–0.92), romosozumab (HR 0.67, 95% CI 0.47–0.95), and denosumab (HR 0.68, 95% CI 0.50–0.85) reduced clinical fracture risk. Five drugs reduced vertebral fracture risk in the PVF ≥ 50% subgroup whereas seven did in the other subgroup. All drugs did not increase withdrawal risk except for parathyroid hormone (1-84) (PTH) (RR 1.9, 95% CI 1.4–2.6) and abaloparatide (RR 1.6, 95% CI 1.2–2.3).
Conclusion
Different effective drugs should be used to prevent fragility fractures according to PMW with or without PVF, and romosozumab is the only one which can reduce clinical and vertebral fractures in both of the two populations. PTH and abaloparatide are less tolerated than placebo whereas the eight other drugs assessed in the study have the same tolerability as placebo.





Similar content being viewed by others
Abbreviations
- PMOW:
-
Postmenopausal osteoporotic women
- PMW:
-
Postmenopausal women
- HR:
-
Hazard ratio
- RR:
-
Risk ratio
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- SUCRA:
-
Surface under the cumulative ranking curve
- RCT:
-
Randomized controlled trial
- PTH:
-
Parathyroid hormone (1-84)
- PVF:
-
Prevalent vertebral fracture
References
Black DM, Rosen CJ (2016) Clinical practice. Postmenopausal osteoporosis. N Engl J Med 374:254–262
Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet 393:364–376
Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, Almasri J, Farah W, Sarigianni M, Muthusamy K, Al NA, Haydour Q, Wang Z, Murad MH (2019) Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab 104:1623–1630
Reginster J, Bianic F, Campbell R, Martin M, Williams SA, Fitzpatrick LA (2019) Abaloparatide for risk reduction of nonvertebral and vertebral fractures in postmenopausal women with osteoporosis: a network meta-analysis. Osteoporos Int 30:1465–1473
Freemantle N, Cooper C, Diez-Perez A, Gitlin M, Radcliffe H, Shepherd S, Roux C (2013) Results of indirect and mixed treatment comparison of fracture efficacy for osteoporosis treatments: a meta-analysis. Osteoporos Int 24:209–217
Liu GF, Wang ZQ, Liu L, Zhang BT, Miao YY, Yu SN (2018) A network meta-analysis on the short-term efficacy and adverse events of different anti-osteoporosis drugs for the treatment of postmenopausal osteoporosis. J Cell Biochem 119:4469–4481
Migliore A, Broccoli S, Massafra U, Cassol M, Frediani B (2013) Ranking antireabsorptive agents to prevent vertebral fractures in postmenopausal osteoporosis by mixed treatment comparison meta-analysis. Eur Rev Med Pharmacol Sci 17:658–667
Zhang L, Pang Y, Shi Y, Xu M, Xu X, Zhang J, Ji L, Zhao D (2015) Indirect comparison of teriparatide, denosumab, and oral bisphosphonates for the prevention of vertebral and nonvertebral fractures in postmenopausal women with osteoporosis. Menopause 22:1021–1025
Messori A, Fadda V, Maratea D, Trippoli S, Marinai C (2014) Anti-reabsorptive agents in women with osteoporosis: determining statistical equivalence according to evidence-based methods. J Endocrinol Investig 37:769–773
Zhou J, Ma X, Wang T, Zhai S (2016) Comparative efficacy of bisphosphonates in short-term fracture prevention for primary osteoporosis: a systematic review with network meta-analyses. Osteoporos Int 27:3289–3300
Ellis AG, Reginster JY, Luo X, Cappelleri JC, Chines A, Sutradhar S, Jansen JP (2014) Bazedoxifene versus oral bisphosphonates for the prevention of nonvertebral fractures in postmenopausal women with osteoporosis at higher risk of fracture: a network meta-analysis. Value Health 17:424–432
Markham A (2019) Romosozumab: first global approval. Drugs 79:471–476
Rachner TD, Hofbauer LC, Gobel A, Tsourdi E (2019) Novel therapies in osteoporosis: PTH-related peptide analogs and inhibitors of sclerostin. J Mol Endocrinol 62:R145–R154
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Dias S, Sutton AJ, Ades AE, Welton NJ (2013) Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Mak 33:607–617
Zhu RS, Kan SL, Ning GZ, Chen LX, Cao ZG, Jiang ZH, Zhang XL, Hu W (2019) Which is the best treatment of osteoporotic vertebral compression fractures: balloon kyphoplasty, percutaneous vertebroplasty, or non-surgical treatment? A Bayesian network meta-analysis. Osteoporos Int 30:287–298
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Zhang YS, Weng WY, Xie BC, Meng Y, Hao YH, Liang YM, Zhou ZK (2018) Glucagon-like peptide-1 receptor agonists and fracture risk: a network meta-analysis of randomized clinical trials. Osteoporos Int 29:2639–2644
Dias S, Welton NJ, Caldwell DM, Ades AE (2010) Checking consistency in mixed treatment comparison meta-analysis. Stat Med 29:932–944
Mills EJ, Thorlund K, Ioannidis JP (2013) Demystifying trial networks and network meta-analysis. BMJ 346:f2914
Tan X, Wen F, Yang W, Xie JY, Ding LL, Mo YX (2019) Comparative efficacy and safety of pharmacological interventions for osteoporosis in postmenopausal women: a network meta-analysis (Chongqing, China). Menopause 26:929–939
Wang G, Sui L, Gai P, Li G, Qi X, Jiang X (2017) The efficacy and safety of vertebral fracture prevention therapies in post-menopausal osteoporosis treatment: Which therapies work best? a network meta-analysis. Bone Joint Res 6:452–463
Saito T, Sterbenz JM, Malay S, Zhong L, MacEachern MP, Chung KC (2017) Effectiveness of anti-osteoporotic drugs to prevent secondary fragility fractures: systematic review and meta-analysis. Osteoporos Int 28:3289–3300
Center JR, Bliuc D, Nguyen TV, Eisman JA (2007) Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 297:387–394
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B (2004) Fracture risk following an osteoporotic fracture. Osteoporos Int 15:175–179
Qaseem A, Forciea MA, McLean RM, Denberg TD (2017) Treatment of low bone density or osteoporosis to prevent fractures in men and women: a clinical practice guideline update from the American College of Physicians. Ann Intern Med 166:818–839
Anastasilakis AD, Polyzos SA, Makras P (2018) Therapy of endocrine disease: denosumab vs bisphosphonates for the treatment of postmenopausal osteoporosis. Eur J Endocrinol 179:R31–R45
Sancar F (2019) Caution with new osteoporosis drug. JAMA 321:1862
Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, Mierzwinski-Urban M, Clifford T, Hutton B, Rabb D (2012) The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care 28:138–144
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A (2017) Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med 377:1417–1427
Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, Hofbauer LC, Lau E, Lewiecki EM, Miyauchi A, Zerbini CA, Milmont CE, Chen L, Maddox J, Meisner PD, Libanati C, Grauer A (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543
Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, Alexandersen P, Zerbini CA, Hu MY, Harris AG, Fitzpatrick LA, Cosman F, Christiansen C (2016) Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 316:722–733
Kendler DL, Marin F, Zerbini C, Russo LA, Greenspan SL, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner-Pammer A, Lespessailles E, Minisola S, Body JJ, Geusens P, Moricke R, Lopez-Romero P (2018) Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet 391:230–240
Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, Wiessing KR, Bolland MJ, Bastin S, Gamble GD (2018) Fracture prevention with zoledronate in older women with osteopenia. N Engl J Med 379:2407–2416
Cosman F (2018) Long-term treatment strategies for postmenopausal osteoporosis. Curr Opin Rheumatol 30:420–426
Lorentzon M (2019) Treating osteoporosis to prevent fractures: current concepts and future developments. J Intern Med 285:381–394
Buckley L, Humphrey MB (2018) Glucocorticoid-induced osteoporosis. N Engl J Med 379:2547–2556
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary materials
Appendix 1
Full search strategy in PubMed, and trials included for quantitative synthesis. (PDF 70 kb)
Appendix 2
Study characteristics and quality assessment. (XLSX 18 kb)
Appendix 3
Heterogeneity assessment for clinical fracture in the subgroup of the proportion of PVF ≥ 50%. (PDF 91 kb)
Appendix 4
Heterogeneity assessment for clinical fracture in the subgroup of the proportion of PVF < 50%. (PDF 149 kb)
Appendix 5
Heterogeneity assessment for tolerability with all trials included. (PDF 151 kb)
Appendix 6
Heterogeneity assessment for vertebral fracture in the subgroup of the proportion of PVF ≥ 50%. (PDF 61 kb)
Appendix 7
Heterogeneity assessment for vertebral fracture in the subgroup of the proportion of PVF < 50%. (PDF 121 kb)
Appendix 8
Inconsistency test for clinical fracture in the subgroup of the proportion of PVF ≥ 50%. (PDF 56 kb)
Appendix 9
Inconsistency test for clinical fracture in the subgroup of the proportion of PVF < 50%. (PDF 84 kb)
Appendix 10
Inconsistency test for tolerability with all trials included. (PDF 89 kb)
Appendix 11
Inconsistency test for vertebral fracture in the subgroup of the proportion of PVF ≥ 50%. (PDF 29 kb)
Appendix 12
Inconsistency test for vertebral fracture in the subgroup of the proportion of PVF < 50%. (PDF 85 kb)
Appendix 13
Network meta-analysis forest plot of vertebral fracture for the subgroup of the proportion of PVF ≥ 50%. (PDF 130 kb)
Appendix 14
Network meta-analysis forest plot of vertebral fracture for the subgroup of the proportion of PVF < 50%. (PDF 173 kb)
Appendix 15
Sensitivity network meta-analysis for clinical fracture in the subgroup of the proportion of PVF < 50%. (PDF 83 kb)
Appendix 16
Sensitivity network meta-analysis for tolerability. (PDF 83 kb)
Appendix 17
Sensitivity network meta-analysis for vertebral fracture in the subgroup of the proportion of PVF < 50%. (PDF 83 kb)
Appendix 18
Comparison-adjusted funnel plots of clinical fracture for the subgroup of the proportion of PVF ≥ 50% (A), clinical fracture for the subgroup of the proportion of PVF < 50% (B), tolerability for all trials (C), vertebral fracture for the subgroup of the proportion of PVF ≥ 50% (D), and vertebral fracture for the subgroup of the proportion of PVF < 50% (E). (PDF 181 kb)
Rights and permissions
About this article
Cite this article
Ding, LL., Wen, F., Wang, H. et al. Osteoporosis drugs for prevention of clinical fracture in white postmenopausal women: a network meta-analysis of survival data. Osteoporos Int 31, 961–971 (2020). https://doi.org/10.1007/s00198-019-05183-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-019-05183-4