Abstract
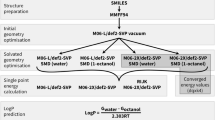
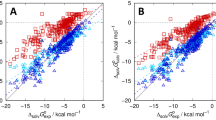
Blind predictions of octanol/water partition coefficients at 298 K for 11 kinase inhibitor fragment like compounds were made for the SAMPL6 challenge. We used the conventional, “untrained”, free energy based approach wherein the octanol/water partition coefficient was computed directly as the difference in solvation free energy in water and 1-octanol. We additionally proposed and used two different forms of a “trained” approach. Physically, the goal of the trained approach is to relate the partition coefficient computed using pure 1-octanol to that using water-saturated 1-octanol. In the first case, we assumed the partition coefficient using water-saturated 1-octanol and pure 1-octanol are linearly correlated. In the second approach, we assume the solvation free energy in water-saturated 1-octanol can be written as a linear combination of the solvation free energy in pure water and 1-octanol. In all cases here, the solvation free energies were computed using electronic structure calculations in the SM12, SM8, and SMD universal solvent models. In the context of the present study, our results in general do not support the additional effort of the trained approach.









Similar content being viewed by others
References
Leo A, Elkins CHD (1971) Partition coefficients and their uses. Chem Rev 71:525–616
Sangster J (1989) Octanol-water partition coefficients of simple organic compounds. J Phys Chem Ref Data 18:1111–1227
Sangster J (1997) Octanol-water partition coefficients: fundamentals and physical chemistry. Wiley, Chichester
Vitha M, Carr PW (2006) The chemical interpretation and practice of linear solvation energy relationships in chromatography. J Chromatogr A 1126:143–194
Sedov IA, Salikov TM, Qian E, Wadawadigi A, Zha O, Acree WE Jr, Abraham MH (2019) Abraham model correlations for solute transfer into 2-methyl-2-butanol based on measured activity coefficient and solubility data at 298.15 K. J Mol Liq 293:111454
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv. Rev 46:3–26
Bhatnagar N, Kamath G, Chelst I, Potoff JJ (2012) Direct calculation of 1-octanol-water partition coefficients from adaptive biasing force molecular dynamics simulations. J Chem Phys 137:014502
Kamath NBG, Potoff JJ (2013) Prediction of 1-octanol-water and air-water partition coefficients for nitro-aromatic compounds from molecular dynamics simulations. Phys Chem Chem Phys 15:6467–6474
Zhang H, Jiang Y, Cui Z, Yin C (2018) Force field benchmark of amino acids. 2. Partition coefficients between water and organic solvents. J Chem Inf Model 58:1669–1681
Nedyalkova MA, Madurga S, Tobiszewski M, Simeonov V (2019) Calculating partition coefficients of organic solvents in octanol/water and octanol/air. J Chem Inf Model 59:2257–2263
Bannan CC, Calabró G, Kyu DY, Mobley DL (2016) Calculating partition coefficients of small molecules in octanol/water and cyclohexane/water. J Chem Theory Comput 12:4015–4024
Kundi V, Ho J (2019) Predicting octanol-water partition coefficients: are quantum mechanical implicit solvent models better than empirical fragment-based methods? J Phys Chem B 123:6810–6822
van der Spoel D, Manzetti S, Zhang H, Klamt A (2019) Prediction of partition coefficients of environmental toxins using computational chemistry methods. ACS Omega 4:13772–13781
Essex JW, Reynolds CA, Richards WG (1989) Relative partition coefficients from partition functions: a theoretical approach to drug transport. J Chem Soc Chem Commun 1152–1154
Essex JW, Reynolds CA, Richards WG (1992) Theoretical determination of partition coefficients. J Am Chem Soc 3634–3639
Michel J, Orsi M, Essex JW (2008) Prediction of partition coefficients by multiscale hybrid atomic-level/coarse-grain simulations. J Phys Chem B 657–660
Jorgensen WL, Briggs JM, Contreras ML (1990) Relative partition coefficients for organic solutes from fluid simulations. J Phys Chem 1683–1686
Ogata K, Hatakeyama M, Nakamura S (2018) Effect of Atomic charges on octanol-water partition coefficient using alchemical free energy calculation. Molecules 23:425
Garrido NM, Queimada AJ, Jorge M, Macedo EA, Economou IG (2009) 1-Octanol/water partition coefficients of n-alkanes from molecular simulation of absolute solvation free energies. J Chem Theory Comput 5:2436–2446
Garrido NM, Economou IG, Queimada AJ, Jorge M, Macedo EA (2012) Prediction of the n-hexane/water and 1-octanol/water partition coefficients for environmentally relevant compounds using molecular simulation. AIChE J 58:1929–1938
Yang L, Ahmed A, Sandler SI (2013) Comparison of two simulation methods to compute solvation free energies and partition coefficients. J Comput Chem 34:284–293
Sørensen JM, Arlt W (eds) (1979) Liquid-liquid equilibrium data collection, Part 1: Binary systems. DECHEMA, Frankfurt am Main
Kristi A, Vesnaver G (1995) Thermodynamic investigation of the effect of octanol-water mutual miscibility on the partitioning and solubility of some guanine derivatives. J Chem Soc Faraday Trans 91:995–998
Tse G, Sandler SI (1994) Determination of infinite dilution activity coefficients and 1-octanol/water partition coefficients of volatile organic pollutants. J Chem Eng Data 39:354–357
Chen B, Siepmann JI (2000) Partitioning of alkane and alcohol solutes between water and (dry or wet) 1-octanol. J Am Chem Soc 122:6464–6467
Chen B, Siepmann JI (2006) Microscopic structure and solvation in dry and wet octanol. J Phys Chem B 110:3555–3563
MacCullum JL, Tieleman DP (2002) Structures of neat and hydrated 1-octanol from computer simulations. J Am Chem Soc 124:15085–15093
Lin S, Sandler SI (1999) Prediction of octanol-water partition coefficients using a group contribution solvent model. Ind Eng Chem Res 38:4081–4091
Marenich AV, Cramer CJ, Truhlar DG (2013) Generalized born solvation model SM12. J Chem Theory Comput 9:609–620
Marenich AV, Olson RM, Kelly CP, Cramer CJ, Truhlar DG (2007) Self-consistent reaction field model for aqueous and nonaqueous solutions based on accurate polarized partial charges. J Chem Theory Comput 3:2011–2033
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Prausnitz JM, Lichtenthaler RN, de Azevedo EG (1986) Molecular thermodynamics of fluid-phase equilibria, 2nd edn. Prentice-Hall Inc., Englewood Cliffs
OECD: Test No. 107: Partition Coefficient (n-octanol/water): Shake Flask Method (1995) https://doi.org/10.1787/9789264069626-en. https://www.oecd-ilibrary.org/content/publication/9789264069626-en
Cramer CJ (2002) Essentials of computational chemistry. Wiley, Chichester
Chipot C, Pohorille A (eds) (2007) Free energy calculations: theory and applications in chemistry and biology, Springer Series in Chemical Physics, vol 86. Springer, New York
Mobley DL, Guthrie JP (2014) FreeSolv: a database of experimental and calculated hydration free energies, with input files. J Comput Aided Mol Des 28:711–720
Paluch AS, Maginn EJ (2013) Predicting the solubility of solid phenanthrene: a combined molecular simulation and group contribution approach. AIChE J 59:2647–2661
Noroozi J, Paluch AS (2017) Microscopic structure and solubility predictions of multifunctional solids in supercritical carbon dioxide: a molecular simulation study. J Phys Chem B 121:1660–1674
Long GE, Dhakal P, Redeker BN, Paluch AS (2019) Using limiting activity coefficients to efficiently evaluate the ability of fixed-charge force fields to model miscible water plus cosolvent mixtures. Mol Simul 45:322–335
Ley RT, Fuerst GB, Redeker BN, Paluch AS (2016) Developing a predictive form of MOSCED for nonelectrolyte solids using molecular simulation: application to acetanilide, acetaminophen, and phenacetin. Ind Eng Chem Res 55:5415–5430
Weininger D (1988) SMILES, a chemical language and information system. 1. Introduction to methodology and encoding rules. J Chem Inf Comput Sci 28:31–36
Daylight Chemical Information Systems, Inc. https://www.daylight.com/. (accessed June 26, 2019)
SAMPL6 logP Challenge Instructions. https://github.com/samplchallenges/SAMPL6/blob/master/logP_challenge_instructions.md. Accessed 11 Jan 2019
Wishart DS, Knox C, Guo AC, Shrivastava S, Hassanali M, Stothard P, Chang Z, Woolsey J (2006) DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res 34:D668–D672
Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2010) DrugBank 3.0: a comprehensive resource for ‘Omics’ research on drugs. Nucleic Acids Res 39:D1035–D1041
Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, Maciejewski A, Arndt D, Wilson M, Neveu V, Tang A, Gabriel G, Ly C, Adamjee S, Dame ZT, Han B, Zhou Y, Wishart DS (2013) DrugBank 4.0: shedding new light on drug metabolism. Nucleic Acids Res 42:D1091–D1097
Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynkkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M (2017) DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46:D1074–D1082
DRUGBANK. https://www.drugbank.ca/. Accessed 11 Jan 2019
DRUGBANK. https://www.drugbank.ca/. Accessed 6 Jan 2020
The Mathworks, Inc., Natick, Massachusetts: MATLAB version 9.4.0.813654 (R2018a) (2018)
fitlm. https://www.mathworks.com/help/stats/fitlm.html. Accessed 3 Jan 2020
Robust Regression – Reduce Outlier Effects. https://www.mathworks.com/help/stats/robust-regression-reduce-outlier-effects.html. Accessed 3 Jan 2020
Marvin JS. https://chemaxon.com/products/marvin-js. Accessed 26 June 2019
Işik M, Levorse D, Mobley DL, Rhodes T, Chodera JD Octanol-water partition coefficient measurements for the SAMPL6 blind prediction challenge. J Comput Aided Mol Des. https://doi.org/10.1007/s10822-019-00271-3
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchinson GR (2011) Open Babel: an open chemical toolbox. J Cheminf 3:33
Open Babel: The Open Source Chemistry Toolbox. http://openbabel.org/wiki/Main_Page. Accessed 26 June 2019
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25:1157–1174
Wang J, Wang W, Kollman PA, Case DA (2006) Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model 25:247–260
Gasteiger J, Marsili M (1978) A new model for calculating atomic charges in molecules. Tetrahedron Lett 34:3181–3184
Zhao Y, Truhlar DG (2008) The M06 theory of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023
Shao Y, Gan Z, Epifanovsky E, Gilbert ATB, Wormit M, Kussmann J, Lange AW, Behn A, Deng J, Feng X, Ghosh D, Goldey M, Horn PR, Jacobson LD, Kaliman I, Khaliullin RZ, Kús T, Landau A, Liu J, Proynov EI, Rhee YM, Richard RM, Rohrdanz MA, Steele RP, Sundstrom EJ, Woodcock HL III, Zimmerman PM, Zuev D, Albrecht B, Alguire E, Austin B, Beran GJO, Bernard YA, Berquist E, Brandhorst K, Bravaya KB, Brown ST, Casanova D, Chang CM, Chen Y, Chien SH, Closser KD, Crittenden DL, Diedenhofen M, DiStasio RA Jr, Dop H, Dutoi AD, Edgar RG, Fatehi S, Fusti-Molnar L, Ghysels A, Golubeva-Zadorozhnaya A, Gomes J, Hanson-Heine MWD, Harbach PHP, Hauser AW, Hohenstein EG, Holden ZC, Jagau TC, Ji H, Kaduk B, Khistyaev K, Kim J, Kim J, King RA, Klunzinger P, Kosenkov D, Kowalczyk T, Krauter CM, Lao KU, Laurent A, Lawler KV, Levchenko SV, Lin CY, Liu F, Livshits E, Lochan RC, Luenser A, Manohar P, Manzer SF, Mao SP, Mardirossian N, Marenich AV, Maurer SA, Mayhall NJ, Oana CM, Olivares-Amaya R, O’Neill DP, Parkhill JA, Perrine TM, Peverati R, Pieniazek PA, Prociuk A, Rehn DR, Rosta E, Russ NJ, Sergueev N, Sharada SM, Sharmaa S, Small DW, Sodt A, Stein T, Stück D, Su YC, Thom AJW, Tsuchimochi T, Vogt L, Vydrov O, Wang T, Watson MA, Wenzel J, White A, Williams CF, Vanovschi V, Yeganeh S, Yost SR, You ZQ, Zhang IY, Zhang X, Zhou Y, Brooks BR, Chan GKL, Chipman DM, Cramer CJ, Goddard WA III, Gordon MS, Hehre WJ, Klamt A, Schaefer HF III, Schmidt MW, Sherrill CD, Truhlar DG, Warshel A, Xua X, Aspuru-Guzik A, Baer R, Bell AT, Besley NA, Chai JD, Dreuw A, Dunietz BD, Furlani TR, Gwaltney SR, Hsu CP, Jung Y, Kong J, Lambrecht DS, Liang W, Ochsenfeld C, Rassolov VA, Slipchenko LV, Subotnik JE, Van Voorhis T, Herbert JM, Krylov AI, Gill PMW, Head-Gordon M (2015) Advances in molecular quantum chemistry contained in the Q-Chem 4 program package. Mol Phys 113:184–215
The Human Metabolome Database (HMB). http://www.hmdb.ca/. Accessed 6 Jan 2020
Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, Fung C, Nikolai L, Lewis M, Coutouly MA, Forsythe I, Tang P, Shrivastava S, Jeroncic K, Stothard P, Amegbey G, Block D, Hau DD, Wagner J, Miniaci J, Clements M, Gebremedhin M, Guo N, Zhang Y, Duggan GE, MacInnis GD, Weljie AM, Dowlatabadi R, Bamforth F, Clive D, Greiner R, Li L, Marrie T, Sykes BD, Vogel HJ, Querengesser L (2007) HMDB: the Human Metabolome Database. Nucleic Acids Res 35:D521–D526
Wishart DS, Knox C, Guo AC, Eisner R, Young N, Gautam B, Hau DD, Psychogios N, Dong E, Bouatra S, Mandal R, Sinelnikov I, Xia J, Jia L, Cruz JA, Lim E, Sobsey CA, Shrivastava S, Huang P, Liu P, Fang L, Peng J, Fradette R, Cheng D, Tzur D, Clements M, Lewis A, De Souza A, Zuniga A, Dawe M, Xiong Y, Clive D, Greiner R, Nazyrova A, Shaykhutdinov R, Li L, Vogel HJ, Forsythe I (2008) HMDB: a knowledgebase for the human metabolome. Nucleic Acids Res 37:D603–D610
Wishart DS, Jewison T, Guo AC, Wilson M, Knox C, Liu Y, Djoumbou Y, Mandal R, Aziat F, Dong E, Bouatra S, Sinelnikov I, Arndt D, Xia J, Liu P, Yallou F, Bjorndahl T, Perez-Pineiro R, Eisner R, Allen F, Neveu V, Greiner R, Scalbert A (2012) HMDB 3.0—The Human Metabolome Database in 2013. Nucleic Acids Res 41:D801–D807
Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vázquez-Fresno R, Sajed T, Johnson D, Li C, Karu N, Sayeeda Z, Lo E, Assempour N, Berjanskii M, Singhal S, Arndt D, Liang Y, Badran H, Grant J, Serra-Cayuela A, Liu Y, Mandal R, Neveu V, Pon A, Knox C, Wilson M, Manach C, Scalbert A (2017) HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res 46:D608–D617
SAMPL6. https://github.com/samplchallenges/SAMPL6/blob/master/physical_properties/logP/analysis/analysis_outputs/StatisticsTables/statistics.csv. Accessed 3 Jan 2020
Winget P, Dolney DM, Giesen DJ, Cramer CJ, Truhlar DG Minnesota Solvent Descriptor Database. https://comp.chem.umn.edu/solvation/mnsddb.pdf. Accessed 13 Nov 2018
QChem 5.1 User Manual: 12.2 Chemical Solvent Models. https://www.q-chem.com/qchem-website/manual/qchem51_manual/sect-solvent.html. Accessed 26 June 2019
Marenich AV, Kelly CP, Thompson JD, Hawkins GD, Chambers CC, Giesen DJ, Winget P, Cramer CJ, Truhlar DG Minnesota Solvation Database - version 2012. http://comp.chem.umn.edu/mnsol. Accessed 13 Nov 2018
Rumble JR (ed) (2019) CRC handbook of chemistry and physics, 100th (internet version 2019) edn. CRC Press/Taylor & Francis, Boca Raton
Ohio Supercomputer Center: Ohio Supercomputer Center (1987). http://osc.edu/ark:/19495/f5s1ph73
Acknowledgements
All of the electronic structure calculations were performed at the Ohio Supercomputer Center (OSC) on the Owens supercomputer [73].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ouimet, J.A., Paluch, A.S. Predicting octanol/water partition coefficients for the SAMPL6 challenge using the SM12, SM8, and SMD solvation models. J Comput Aided Mol Des 34, 575–588 (2020). https://doi.org/10.1007/s10822-020-00293-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-020-00293-2