Abstract
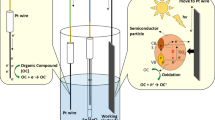
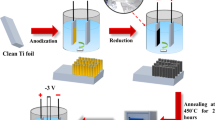
The synthesis of a self-doped TiO2 nanotubes (SD-TNT) electrode and its potential applicability as a sensing platform electrode for voltammetric determination of methylene blue (MB) dye is reported. Highly ordered TiO2 nanotubes vertically oriented to the titanium surface were grown and the electrochemical doping did not change the morphology of the TNT. Raman spectroscopy demonstrated that the electrochemical doping of TNT promoted a blue shift of the Eg1 mode (from 152 to 158 cm−1) which results in an increased conductivity due to the phonon confinement resultant from the formation of non-stoichiometry Ti3+ donor states. The structural changes allowed the application of SD-TNT as a voltammetric sensor of MB without any UV irradiation. The SD-TNT electrode allowed an efficient quantification of MB, with a limit of detection of 0.48 and 0.90 μmol L−1 when considered the oxidation and reduction peak, respectively. Then, SD-TNT electrode was applied to the monitoring of MB concentration during electro-Fenton oxidation. MB removal fitted well with pseudo-first-order kinetics, suggesting a constant formation of •OH radicals and oxidation of MB. These results revealed that the SD-TNT is a versatile, simple, and promising material for the voltammetric determination of synthetic dyes.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Garcia-Segura S, Brillas E (2017) Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J Photochem Photobiol C Photochem Rev 31:1–35
Brillas E, Martínez-Huitle CA (2015) Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl Catal B Environ 166–167:603–643
Sala M, Gutiérrez-Bouzán MC (2012) Electrochemical techniques in textile processes and wastewater treatment. Int J Photoenergy 2012:12
Almeida LC, Silva BF, Zanoni MVB (2014) Combined photoelectrocatalytic/electro-Fenton process using a Pt/TiO2NTs photoanode for enhanced degradation of an azo dye: a mechanistic study. J Electroanal Chem 734:43–52
Labiadh L, Barbucci A, Cerisola G et al (2015) Role of anode material on the electrochemical oxidation of methyl orange. J Solid State Electrochem 19:3177–3183
Labiadh L, Barbucci A, Carpanese MP et al (2017) Direct and indirect electrochemical oxidation of indigo carmine using PbO2 and TiRuSnO2. J Solid State Electrochem 21:2167–2175
Pereira JF, Figueiredo RS, Ponce-de-León C, Bertazzoli R (2016) Platinum-free lead dioxide electrode for electrooxidation of organic compounds. J Solid State Electrochem 20:1167–1173
Hu X, Yu Y, Yang L (2015) Electrocatalytic activity of Ce-PbO2/C anode for acid red B reduction in aqueous solution. J Solid State Electrochem 19:1599–1609
Almeida LC, Garcia-Segura S, Arias C, Bocchi N, Brillas E (2012) Electrochemical mineralization of the azo dye Acid Red 29 (Chromotrope 2R) by photoelectro-Fenton process. Chemosphere 89(6):751–758
Yang W, Zhou M, Oturan N et al (2019) Electrocatalytic destruction of pharmaceutical imatinib by electro-Fenton process with graphene-based cathode. Electrochim Acta 305:285–294
Garcia-Segura S, Almeida LC, Bocchi N, Brillas E (2011) Solar photoelectro-Fenton degradation of the herbicide 4-chloro-2-methylphenoxyacetic acid optimized by response surface methodology. J Hazard Mater 194:109–118
Almeida LC, Garcia-Segura S, Bocchi N, Brillas E (2011) Solar photoelectro-Fenton degradation of paracetamol using a flow plant with a Pt/air-diffusion cell coupled with a compound parabolic collector: process optimization by response surface methodology. Appl Catal B Environ 103:21–30
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on fenton’s reaction chemistry. Chem Rev 109:6570–6631
Diagne M, Oturan N, Oturan MA (2007) Removal of methyl parathion from water by electrochemically generated Fenton’s reagent. Chemosphere 66(5):841–848
Dean JR, Wade G, Barnabas IJ (1996) Determination of triazine herbicides in environmental samples. J Chromatogr A 733:295–335
Tadeo JL, Sanchez-Brunete C, García-Valcarcel AI, Martínez L, Pérez RA (1996) Determination of cereal herbicide residues in environmental samples by gas chromatography. J Chromatogr A 754(1-2):347–365
Zeid AM, Kaji N, Nasr JJM, Belal FF, Baba Y, Walash MI (2017) Stacking-cyclodextrin-microchip electrokinetic chromatographic determination of gabapentinoid drugs in pharmaceutical and biological matrices. J Chromatogr A 1503:65–75
Culzoni MJ, Schenone AV, Llamas NE, Garrido M, di Nezio MS, Band BS, Goicoechea HC (2009) Fast chromatographic method for the determination of dyes in beverages by using high performance liquid chromatography-diode array detection data and second order algorithms. J Chromatogr A 1216(42):7063–7070
Bessegato GG, Zanoni MVB, Tremiliosi-Filho G, Lindino CA (2019) Evidences of the electrochemical production of sulfate radicals at cathodically polarized TiO2 nanotubes electrodes. Electrocatalysis 10:272–276
Uslu B, Ozkan SA (2011) Electroanalytical methods for the determination of pharmaceuticals: a review of recent trends and developments. Anal Lett 44:2644–2702
Ola O, Maroto-Valer MM (2015) Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J Photochem Photobiol C Photochem Rev 24:16–42
Liu H, Liu G, Fan J, Zhou Q, Zhou H, Zhang N, Hou Z, Zhang M, He Z (2011) Photoelectrocatalytic degradation of 4,4′-dibromobiphenyl in aqueous solution on TiO2 and doped TiO2 nanotube arrays. Chemosphere 82(1):43–47
Liu H, Cao X, Liu G, Wang Y, Zhang N, Li T, Tough R (2013) Photoelectrocatalytic degradation of triclosan on TiO 2 nanotube arrays and toxicity change. Chemosphere 93(1):160–165
Oriol R, Sirés I, Brillas E, De Andrade AR (2019) A hybrid photoelectrocatalytic/photoelectro-Fenton treatment of indigo carmine in acidic aqueous solution using TiO2 nanotube arrays as photoanode. J Electroanal Chem 847:113088
Bessegato GG, De Almeida LC, Ferreira SLC, Zanoni MVB (2019) Experimental design as a tool for parameter optimization of photoelectrocatalytic degradation of a textile dye. J Environ Chem Eng 7:103264
Cardoso JC, Stulp S, de Brito JF et al (2018) MOFs based on ZIF-8 deposited on TiO2 nanotubes increase the surface adsorption of CO2 and its photoelectrocatalytic reduction to alcohols in aqueous media. Appl Catal B Environ 225:563–573
de Brito JF, Hudari FF, Zanoni MVB (2018) Photoelectrocatalytic performance of nanostructured p-n junction NtTiO2/NsCuO electrode in the selective conversion of CO2 to methanol at low bias potentials. J CO2 Util 24:81–88
Guaraldo TT, Gonçales VR, Silva BF et al (2016) Hydrogen production and simultaneous photoelectrocatalytic pollutant oxidation using a TiO2/WO3 nanostructured photoanode under visible light irradiation. J Electroanal Chem 765:188–196
Frank SN, Bard AJ (1975) Semiconductor electrodes. II. Electrochemistry at n-type TiO2 electrodes in acetonitrile solutions. J Am Chem Soc 97:7427–7433
Feng C, Xu G, Liu H et al (2014) Glucose biosensors based on Ag nanoparticles modified TiO2 nanotube arrays. J Solid State Electrochem 18:163–171
Ardakani MM, Talebi A, Naeimi H et al (2009) Fabrication of modified TiO2 nanoparticle carbon paste electrode for simultaneous determination of dopamine, uric acid, and l-cysteine. J Solid State Electrochem 13:1433–1440
Li H, Li M, Guo W et al (2014) Electrochemical application of titanium dioxide nanoparticle/gold nanoparticle/multiwalled carbon nanotube nanocomposites for nonenzymatic detection of ascorbic acid. J Solid State Electrochem 18:477–485
Grimes CA, Mor GK (2009) TiO2 nanotube arrays: synthesis, properties, and applications. Springer, New York
Cardoso JC, Bessegato GG, de Brito JF et al (2017) Electrochemistry: a powerful tool for preparation of semiconductor materials for decontamination of organic and inorganic pollutants, disinfection, and CO2 reduction. Recent Adv Complex Funct Mater 239–269
Bessegato GG, Hudari FF, Zanoni MVB (2017) Self-doped TiO2 nanotube electrodes: a powerful tool as a sensor platform for electroanalytical applications. Electrochim Acta 235:527–533
Hudari FF, Bessegato GG, Bedatty Fernandes FC, Zanoni MVB, Bueno PR (2018) Reagentless detection of low-molecular-weight triamterene using self-doped TiO2 nanotubes. Anal Chem 90(12):7651–7658
Almeida LC, Zanoni MVB (2014) Decoration of Ti/TiO2 nanotubes with Pt nanoparticles for enhanced UV-Vis light absorption in photoelectrocatalytic process. J Braz Chem Soc 25:579–588
Magro C, Mateus EP, Paz-Garcia JM et al (2019) Electronic tongue coupled to an electrochemical flow reactor for emerging organic contaminants real time monitoring. Sensors 19:5349
Long GL, Winefordner JD (1983) Limit of detection a closer look at the IUPAC definition. Anal Chem 55:712A–724A
Zhu WD, Wang CW, Chen JB et al (2014) Enhanced field emission from Ti 3+ self-doped TiO 2 nanotube arrays synthesized by a facile cathodic reduction process. Appl Surf Sci 301:525–529
Hu M, Cao Y, Li Z et al (2017) Ti 3+ self-doped mesoporous black TiO 2 /SiO 2 nanocomposite as remarkable visible light photocatalyst. Appl Surf Sci 426:734–744
Macak JM, Gong BG, Hueppe M, Schmuki P (2007) Filling of TiO2 nanotubes by self-doping and electrodeposition. Adv Mater 19:3027–3031
(2002) Allen J. Bard and Larry R. Faulkner, Electrochemical methods: fundamentals and applications, New York: Wiley, 2001, 2nd ed. Russ J Electrochem 38:1364–1365
Tonlé IK, Ngameni E, Tcheumi HL, Tchiéda V, Carteret C, Walcarius A (2008) Sorption of methylene blue on an organoclay bearing thiol groups and application to electrochemical sensing of the dye. Talanta 74(4):489–497
Nekoueian K, Jafari S, Amiri M, Sillanpaa M (2018) Pre-adsorbed methylene blue at carbon-modified TiO2 electrode: application for lead sensing in water. IEEE Sensors J 18:9477–9485
Hosoi T, Kodama D, Kohno Y et al (2014) Electrochemical response of diamond electrode to methylene blue in aqueous solution. J Surf Finish Soc Japan 65:104–107
Almeida LC, Silva BF, Zanoni MVB (2015) Photoelectrocatalytic/photoelectro-Fenton coupling system using a nanostructured photoanode for the oxidation of a textile dye: kinetics study and oxidation pathway. Chemosphere 136:63–71
Bessegato GG, Cardoso JC, da Silva BF, Zanoni MVB (2016) Combination of photoelectrocatalysis and ozonation: a novel and powerful approach applied in acid yellow 1 mineralization. Appl Catal B Environ 180:161–168
Farhat A, Keller J, Tait S, Radjenovic J (2015) Removal of persistent organic contaminants by electrochemically activated sulfate. Environ Sci Technol 49(24):14326–14333
Li J, Song D, Du K et al (2019) Performance of graphite felt as a cathode and anode in the electro-Fenton process. RSC Adv 9:38345–38354
Panizza M, Barbucci A, Ricotti R, Cerisola G (2007) Electrochemical degradation of methylene blue. Sep Purif Technol 54:382–387
Erdem A, Kerman K, Meric B et al (2000) Novel hybridization indicator methylene blue for the electrochemical detection of short DNA sequences related to the hepatitis B virus. Anal Chim Acta 422:139–149
Tani A, Thomson AJ, Butt JN (2001) Methylene blue as an electrochemical discriminator of single-and double-stranded oligonucleotides immobilised on gold substrates. Analyst 126(10):1756–1759
Acknowledgments
The authors are grateful to the Chemistry graduate program of Londrina State University (UEL) and to the LARX, LabESPEC, and LMEM research laboratories at UEL for analysis.
Funding
This study was funded by the Brazilian Research Agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 179 kb)
Rights and permissions
About this article
Cite this article
Soto, P.C., Salamanca-Neto, C.A.R., Moraes, J.T. et al. A novel sensing platform based on self-doped TiO2 nanotubes for methylene blue dye electrochemical monitoring during its electro-Fenton degradation. J Solid State Electrochem 24, 1951–1959 (2020). https://doi.org/10.1007/s10008-020-04509-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04509-1