Abstract
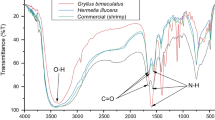
Chitin is the second most widely available natural fiber with diverse applications. Insect chitin is gaining popularity over the last decade. Black soldier fly is a widely known insect with waste management potential. In this study, chitin was isolated from Black soldier fly (Hermetia illucens) pupae exuvia (BSFE) and imago (BSFI). The chitin content was found to be 9% and 23% for pupae exuviae and imago respectively. Both the chitins were α-chitin. The degree of acetylation (DA) confirms that BSFE chitin has higher purity than BSFI chitin. The BSFE chitin is more amorphous than BSFI chitin. The crystallinity index (CrI) for BSFE and BSFI chitin was 25.20% and 49.4%, respectively. Both the chitins had good thermal stability with a maximum degradation (DTGMax) of BSFI and BSFE chitin at 363 °C and 371 °C, respectively. The Brunauer–Emmett–Teller (BET) study showed that chitin from BSFI was mesoporous with well defined cylindrical pore channels while the chitin from BSFE was non-porous. The surface area of BSFE and BSFI chitin was 1.63 and 23.00 m2/g respectively. Both the chitin had a smooth microfibrillar structure with repeating units. Based on the physicochemical characteristics of the BSF-derived chitin it can find promising commercial applications in tissue engineering, textile industry and as an adsorbent in water and wastewater treatment.






Similar content being viewed by others
References
Kaya M, Sofi K, Sargin I, Mujtaba M (2016) Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr Polym 145:64–70. https://doi.org/10.1016/j.carbpol.2016.03.010
Martínez JP, Falomir MP, Gozalbo D (2014) Chitin: A structural biopolysaccharide with multiple applications. eLS 1–10. https://doi.org/10.1002/9780470015902.a0000694.pub3
Bhatnagar A, Sillanpää M (2009) Applications of chitin- and chitosan-derivatives for the detoxification of water and wastewater—a short review. Adv Colloid Interface Sci 152:26–38. https://doi.org/10.1016/j.cis.2009.09.003
Rinaudo M (2006) Chitin and chitosan: properties and applications. Prog Polym Sci 31:603–632. https://doi.org/10.1016/j.progpolymsci.2006.06.001
Deepthi S, Venkatesan J, Kim SK et al (2016) An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int J Biol Macromol 93:1338–1353. https://doi.org/10.1016/j.ijbiomac.2016.03.041
Krajewska B (2004) Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol 35:126–139. https://doi.org/10.1016/j.enzmictec.2003.12.013
Jayakumar R, Menon D, Manzoor K et al (2010) Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr Polym 82:227–232. https://doi.org/10.1016/j.carbpol.2010.04.074
Boonlertnirun S, Boonraung C, Suvanasara R (2008) Application of chitosan in rice production. J Met Mater Miner 18:47–52
Khor E, Lim LY (2003) Implantable applications of chitin and chitosan. Biomaterials 24:2339–2349. https://doi.org/10.1016/S0142-9612(03)00026-7
Hamed I, Özogul F, Regenstein JM (2016) Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): a review. Trends Food Sci Technol 48:40–50. https://doi.org/10.1016/j.tifs.2015.11.007
Ritter-Jones Marsha (2016) Sarah Najjar KMA. HHS Public Access. 1848:3047–3054. https://doi.org/10.1016/j.bbamem.2015.02.010.Cationic
Usman A, Zia KM, Zuber M et al (2016) Chitin and chitosan based polyurethanes: a review of recent advances and prospective biomedical applications. Int J Biol Macromol 86:630–645. https://doi.org/10.1016/j.ijbiomac.2016.02.004
Ravi Kumar MNV (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–27. https://doi.org/10.1016/S1381-5148(00)00038-9
Roberts GAF (2015) Preparation of Chitin and Chitosan. Chitin Chemistry. Macmillan Education UK, London, pp 54–84
Liu S, Sun J, Yu L et al (2012) Extraction and characterization of chitin from the beetle Holotrichia parallela motschulsky. Molecules 17:4604–4611. https://doi.org/10.3390/molecules17044604
Kong B-G, Jang M-K, Nah J-W et al (2004) Physicochemical characterization of?-chitin,?-chitin, and?-chitin separated from natural resources. J Polym Sci A 42:3423–3432. https://doi.org/10.1002/pola.20176
Merzendorfer H (2003) Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206:4393–4412. https://doi.org/10.1242/jeb.00709
Watson DW, Newton L, Sheppard C, Gary B, Dove R (2005) Using the black soldier fly, Hermetia illucens as a value added tool for the management of swine manure. J Korean Entomol Appl Sci 17:17
Vogel H, Müller A, Heckel DG et al (2018) Nutritional immunology: diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev Comp Immunol 78:141–148. https://doi.org/10.1016/j.dci.2017.09.008
Lord WD, Goff ML, Adkins TR, Haskell NH (2015) The black soldier fly Hermetia illucens (Diptera: stratiomyidae) as a potential measure of human postmortem interval: observations and case histories. J Forensic Sci 39:13587J. https://doi.org/10.1520/jfs13587j
Oonincx DGAB, van Huis A, van Loon JJA (2015) Nutrient utilisation by black soldier flies fed with chicken, pig, or cow manure. J Insects Food Feed 1:131–139. https://doi.org/10.3920/jiff2014.0023
Purkayastha D, Sarkar S, Roy P, Kazmi A (2017) Isolation and morphological study of ecologically-important insect “Hermetia illucens” collected from Roorkee compost plant. Pollution 3:453–459. https://doi.org/10.7508/pj.2017.03
Diener S, Zurbrügg C, Tockner K (2009) Conversion of organic material by black soldier fly larvae: establishing optimal feeding rates. Waste Manag Res 27:603–610. https://doi.org/10.1177/0734242X09103838
Banks IJ, Gibson WT, Cameron MM (2014) Growth rates of black soldier fly larvae fed on fresh human faeces and their implication for improving sanitation. Trop Med Int Heal 19:14–22. https://doi.org/10.1111/tmi.12228
Lindström A, Lalander C, Diener S et al (2013) Faecal sludge management with the larvae of the black soldier fly (Hermetia illucens)—from a hygiene aspect. Sci Total Environ 458–460:312–318. https://doi.org/10.1016/j.scitotenv.2013.04.033
Lalander CH, Fidjeland J, Diener S et al (2014) High waste-to-biomass conversion and efficient Salmonella spp. reduction using black soldier fly for waste recycling. Agron Sustain Dev 35:261–271. https://doi.org/10.1007/s13593-014-0235-4
Debasree P, Sudipta S, Kazmi AA et al (2017) Effect of environmental parameters on the treatment of human fecal waste by black soldier fly larvae. 4th International Faecal Sludge Management Conference. Chennai, India, pp 73–74
Lalander C, Diener S, Zurbrügg C, Vinnerås B (2019) Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J Clean Prod 208:211–219. https://doi.org/10.1016/j.jclepro.2018.10.017
Adeniyi OV, Folorunsho C (2015) Performance of Clarias gariepinus (Burchell, 1822) fed dietary levels of black soldier fly, Hermetia illucens (Linnaeus, 1758) prepupae meal as a protein supplement. Int J Res Fish Aquac 5:89–93
Cullere M, Tasoniero G, Giaccone V et al (2016) Black soldier fly as dietary protein source for broiler quails: apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 10:1923–1930. https://doi.org/10.1017/S1751731116001270
Belforti M, Gasco L, Rotolo L et al (2015) Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim Feed Sci Technol 209:211–218. https://doi.org/10.1016/j.anifeedsci.2015.08.006
Li W, Zheng L, Liu Y et al (2015) Simultaneous utilization of glucose and xylose for lipid accumulation in black soldier fly. Biotechnol Biofuels 8:117. https://doi.org/10.1186/s13068-015-0306-z
Shakil Rana KM, Salam MA, Hashem S, Ariful Islam M (2015) Development of black soldier fly larvae production technique as an alternate fish feed. Int J Res Fish Aquac 5:41–47
Sealey WM, Gaylord TG, Barrows FT et al (2011) Sensory analysis of rainbow trout, oncorhynchus mykiss, fed enriched black soldier fly prepupae, Hermetia illucens. J World Aquac Soc 42:34–45. https://doi.org/10.1111/j.1749-7345.2010.00441.x
Deboosere S, Ovyn A, De Smet S et al (2016) Nutritional composition of black soldier fly (Hermetia illucens) prepupae reared on different organic waste substrates. J Sci Food Agric 97:2594–2600. https://doi.org/10.1002/jsfa.8081
Tschirner M, Simon A (2015) Influence of different growing substrates and processing on the nutrient composition of black soldier fly larvae destined for animal feed. J Insects Food Feed 1:249–259. https://doi.org/10.3920/jiff2014.0008
Kozlova AA, Brodskii ES, Pavlov DS et al (2016) Characteristics of lipid fractions of larvae of the black soldier fly Hermetia illucens. Dokl Biochem Biophys 468:209–212. https://doi.org/10.1134/s1607672916030145
Waśko A, Bulak P, Polak-Berecka M et al (2016) The first report of the physicochemical structure of chitin isolated from Hermetia illucens. Int J Biol Macromol 92:316–320. https://doi.org/10.1016/j.ijbiomac.2016.07.038
Caligiani A, Marseglia A, Leni G et al (2018) Composition of black soldier fly prepupae and systematic approaches for extraction and fractionation of proteins, lipids and chitin. Food Res Int 105:812–820. https://doi.org/10.1016/j.foodres.2017.12.012
Wu CS, Wang SS (2018) Bio-Based electrospun nanofiber of polyhydroxyalkanoate modified with black soldier fly’s pupa shell with antibacterial and cytocompatibility properties. ACS Appl Mater Interfaces 10:42127–42135. https://doi.org/10.1021/acsami.8b16606
Sheppard DC, Tomberlin JK, Joyce JA et al (2002) Rearing Methods for the Black Soldier Fly (Diptera: stratiomyidae). J Med Entomol 39:695–698. https://doi.org/10.1603/0022-2585-39.4.695
Draczynski Z (2008) Honeybee corpses as an available source of chitin. J Appl Polym Sci 109:1974–1981. https://doi.org/10.1002/app.28356
Xu J, McCarthy SP, Gross RA, Kaplan DL (1996) Chitosan film acylation and effects on biodegradability. Macromolecules 29:3436–3440. https://doi.org/10.1021/ma951638b
Guinesi LS, Cavalheiro ÉTG (2006) The use of DSC curves to determine the acetylation degree of chitin/chitosan samples. Thermochim Acta 444:128–133. https://doi.org/10.1016/j.tca.2006.03.003
Kasaai MR (2010) Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: a review. Carbohydr Polym 79:801–810. https://doi.org/10.1016/j.carbpol.2009.10.051
Zhang M, Haga A, Sekiguchi H, Hirano S (2000) Structure of insect chitin isolated from beetle larva cuticle and silkworm (Bombyx mori) pupa exuvia. Int J Biol Macromol 27:99–105. https://doi.org/10.1016/S0141-8130(99)00123-3
Sajomsang W, Gonil P (2010) Preparation and characterization of α-chitin from cicada sloughs. Mater Sci Eng 30:357–363. https://doi.org/10.1016/j.msec.2009.11.014
Sagheer FAA, Al-Sughayer MA, Muslim S, Elsabee MZ (2009) Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr Polym 77:410–419. https://doi.org/10.1016/j.carbpol.2009.01.032
Baran T, Mujtaba M, Duman F et al (2017) An inclusive physicochemical comparison of natural and synthetic chitin films. Int J Biol Macromol 106:1062–1070. https://doi.org/10.1016/j.ijbiomac.2017.08.108
Majtán J, Bíliková K, Markovič O et al (2007) Isolation and characterization of chitin from bumblebee (Bombus terrestris). Int J Biol Macromol 40:237–241. https://doi.org/10.1016/j.ijbiomac.2006.07.010
Kaya M, Erdogan S, Mol A, Baran T (2015) Comparison of chitin structures isolated from seven Orthoptera species. Int J Biol Macromol 72:797–805. https://doi.org/10.1016/j.ijbiomac.2014.09.034
Paulino AT, Simionato JI, Garcia JC, Nozaki J (2006) Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr Polym 64:98–103. https://doi.org/10.1016/j.carbpol.2005.10.032
Quan W-Y, Dong J-J, Ou C-Y et al (2010) Effect of cupric ion on thermal degradation of quaternized chitosan. Carbohydr Polym 81:182–187. https://doi.org/10.1016/j.carbpol.2010.02.049
Iqbal MS, Akbar J, Saghir S et al (2011) Thermal studies of plant carbohydrate polymer hydrogels. Carbohydr Polym 86:1775–1783. https://doi.org/10.1016/j.carbpol.2011.07.020
Asaroglu M, Baran T, Sezen G et al (2014) Extraction and characterization of α-Chitin and Chitosan from six different aquatic invertebrates. Food Biophys 9:145–157. https://doi.org/10.1007/s11483-013-9327-y
Kaya M, Baran T, Erdoʇan S et al (2014) Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Mater Sci Eng, C 45:72–81. https://doi.org/10.1016/j.msec.2014.09.004
Union I, Pure OF, Chemistry A (1985) Reporting physisorption data for. Area 57:603–619
Hwang N, Barron AR (2001) BET surface area analysis of nanoparticles. J Hist Philos 39:445–446. https://doi.org/10.1353/hph.2003.0120
Aranaz I, Mengibar M, Harris R, Panos I, Miralles B, Acosta N, Galed G, Heras A, Aranaz I et al (2009) Functional characterization of chitin and chitosan. Curr Chem Biol 3:203–230
Wang G, Xin Y, Uyama H (2015) Facile fabrication of mesoporous poly(ethylene-co-vinyl alcohol)/chitosan blend monoliths. Carbohydr Polym 132:345–350. https://doi.org/10.1016/j.carbpol.2015.06.040
Zazycki MA, Borba PA, Silva RNF et al (2019) Chitin derived biochar as an alternative adsorbent to treat colored effluents containing methyl violet dye. Adv Powder Technol 30:1494–1503. https://doi.org/10.1016/j.apt.2019.04.026
Côrtes LN, Tanabe EH, Bertuol DA, Dotto GL (2015) Biosorption of gold from computer microprocessor leachate solutions using chitin. Waste Manag 45:272–279. https://doi.org/10.1016/j.wasman.2015.07.016
Kaya M, Akyuz B, Bulut E et al (2016) Chitosan nanofiber production from Drosophila by electrospinning. Int J Biol Macromol 92:49–55. https://doi.org/10.1016/j.ijbiomac.2016.07.021
Acknowledgements
This research was funded by the Department of Biotechnology (Govt. of India), Biotechnology Industry Research Assistance Council (BIRAC) and The Bill and Melinda Gates Foundation (BMGF) under the grant number BIRAC/GCI/0066/02/13-RTTC and Ministry of Human Resource Development (MHRD), Government of India under Swachhta action plan (SAP), grant number ICSR/SAP/2018/SN-1. The first author of this article wants to thank Ministry of Human Resource Development (MHRD) for providing scholarship for carrying out her doctoral research work. The authors want to thank Prof. Deepak Bornare (Head (operations), Center for Analytical Research & Studies, MIT, Aurangabad (India)) for helping with the FTIR sample analysis. The authors also want to thank Mr. Adarsh Kumar of Materials Resource Efficiency Division, CSIR-Indian Institute of Petroleum, Dehradun, India for helping with the BET isotherm studies. The authors are grateful to the anonymous reviewer for valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Purkayastha, D., Sarkar, S. Physicochemical Structure Analysis of Chitin Extracted from Pupa Exuviae and Dead Imago of Wild Black Soldier Fly (Hermetia illucens). J Polym Environ 28, 445–457 (2020). https://doi.org/10.1007/s10924-019-01620-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01620-x