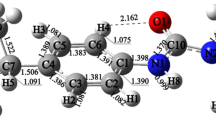
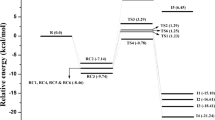
Abstract
Diuron is a phenyl urea herbicide used to control weeds in agricultural lands. The degradation of diuron in the atmosphere takes place dominantly via reaction with OH radicals. In this work, the OH addition reaction of diuron has been studied by using density functional theory methods M06-2X, ωB97X-D and MPWB1K with 6-31G(d,p) basis set. The calculated thermochemical parameters show that OH addition reaction occurs favourably at C2 position of diuron. The rate constant is calculated for the favourable initial reaction pathway by using canonical variational transition state theory with small curvature tunnelling (SCT) correction over the temperature range of 200–1000 K. The reaction of initially formed diuron-OH adduct intermediate with O2 leads to the formation of peroxy radical intermediate. The reaction of peroxy radical intermediate with HO2 and NOx (x = 1, 2) radicals is studied in detail. The results obtained from time-dependent density functional theory (TDDFT) calculations show that the intermediates and products formed from oxidation of diuron can be easily photolyzed in the sunlight. This study provides thermodynamical and kinetic data for the atmospheric oxidation of diuron by OH radical addition reaction and demonstrates the atmospheric chemistry of diuron and its derivatives.














Similar content being viewed by others
References
Amine-Khodja A, Boulkamh A, Boule P (2004) Photochemical behaviour of phenylurea herbicides. Photochem Photobiol Sci 3:145–156
Bai F-Y, Wang X, Sun Y-Q, Pan X-M (2015) Atmospheric chemistry of alkyl iodides: theoretical studies on the mechanisms and kinetics of CH 3 I/C 2 H 5 I+ NO 3 reactions. RSC Adv 5:88087–88095
Baruah SD, Gour NK, Sarma PJ, Deka RC (2018) OH-initiated mechanistic pathways and kinetics of camphene and fate of product radical: a DFT approach. Environ Sci Pollut Res 25:2147–2156
Canle López M, Fernandez MI, Rodríguez S, Santaballa JA, Steenken S, Vulliet E (2005) Mechanisms of direct and TiO2-photocatalysed UV degradation of phenylurea herbicides. ChemPhysChem 6:2064–2074
Chai J-D, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Chang P-L, Hsieh M-M, Chiu T-C (2016) Recent advances in the determination of pesticides in environmental samples by capillary electrophoresis. Int J Environ Res Public Health 13:409
Chiron S, Fernandez-Alba A, Rodriguez A, Garcia-Calvo E (2000) Pesticide chemical oxidation: state-of-the-art. Water Res 34:366–377
Djebbar K, Sehili T, Mazellier P, De Laat J (2003) Phototransformation of diuron in aqueous solution by UV irradiation in the absence and in the presence of H2O2. Environ Technol 24:479–489
Esposito E, Paulillo SM, Manfio GP (1998) Biodegradation of the herbicide diuron in soil by indigenous actinomycetes. Chemosphere 37:541–548
Fingler S, Mendaš G, Dvoršćak M, Stipičević S, Vasilić Ž, Drevenkar V (2017) Herbicide micropollutants in surface, ground and drinking waters within and near the area of Zagreb, Croatia. Environ Sci Pollut Res 24:11017–11030
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (1971): Gaussian 09, Revision A. 02; Gaussian, Inc: Wallingford, CT, 2009. Google Scholar
Fukui K (1997) The path of chemical reactions—the IRC approach, Frontier orbitals and reaction paths: selected papers of Kenichi Fukui. World Scientific, pp. 471–476
Glotfelty DE (1978) The atmosphere as a sink for applied pesticides. J Air Pollut Control Assoc 28:917–921
Gnanaprakasam M, Sandhiya L, Senthilkumar K (2017) A theoretical investigation on the mechanism and kinetics of the gas-phase reaction of naphthalene with OH radical. Theor Chem Accounts 136:131
Gnanaprakasam M, Sandhiya L, Senthilkumar K (2018) Mechanism and kinetics of the oxidation of dimethyl carbonate by hydroxyl radical in the atmosphere. Environ Sci Pollut Res:1–11
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction path following. J Chem Phys 90:2154–2161
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Gross EK, Dobson J, Petersilka M (1996): Density functional theory of time-dependent phenomena, density functional theory II. Springer, pp. 81–172
Guzzella L, Capri E, Di Corcia A, Barra Caracciolo A, Giuliano G (2006) Fate of diuron and linuron in a field lysimeter experiment. J Environ Qual 35:312–323
Hussain S, Arshad M, Springael D, SøRensen SR, Bending GD, Devers-Lamrani M, Maqbool Z, Martin-Laurent F (2015) Abiotic and biotic processes governing the fate of phenylurea herbicides in soils: a review. Crit Rev Environ Sci Technol 45:1947–1998
Jayaraj R, Megha P, Sreedev P (2016) Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip Toxicol 9:90–100
Jirkovský J, Faure V, Boule P (1997) Photolysis of diuron. Pestic Sci 50:42–52
Kabanda MM, Serobatse KR (2018) A DFT study on the addition and abstraction reactions of thiourea with hydroxyl radical. J Sulfur Chem 39:23–46
Kovács K, He S, Mile V, Csay T, Takács E, Wojnárovits L (2015) Ionizing radiation induced degradation of diuron in dilute aqueous solution. Chem Cent J 9:21
Krieger R (2010) Hayes’ handbook of pesticide toxicology, 1. Academic press
Malato S, Cáceres J, Fernández-Alba A, Piedra L, Hernando M, Agüera A, Vial J (2003) Photocatalytic treatment of diuron by solar photocatalysis: evaluation of main intermediates and toxicity. Environ Sci Technol 37:2516–2524
Manonmani G, Sandhiya L, Senthilkumar K (2019) Mechanism and kinetics of diuron oxidation initiated by hydroxyl radical: hydrogen and chlorine atom abstraction reactions. J Phys Chem A 123:8954–8967
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113:6378–6396
Mazellier P, Jirkovsky J, Bolte M (1997) Degradation of diuron photoinduced by iron (III) in aqueous solution. Pestic Sci 49:259–267
Montgomery JA Jr, Frisch MJ, Ochterski JW, Petersson GA (2000) A complete basis set model chemistry VII. Use of the minimum population localization method. J Chem Phys 112:6532–6542
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) Using redundant internal coordinates to optimize equilibrium geometries and transition states. J Comput Chem 17:49–56
Pimentel D (1995) Amounts of pesticides reaching target pests: environmental impacts and ethics. J Agric Environ Ethics 8:17–29
Planas C, Caixach J, Santos F, Rivera J (1997) Occurrence of pesticides in Spanish surface waters. Analysis by high resolution gas chromatography coupled to mass spectrometry. Chemosphere 34:2393–2406
Polcaro AM, Mascia M, Palmas S, Vacca A (2004) Electrochemical degradation of diuron and dichloroaniline at BDD electrode. Electrochim Acta 49:649–656
Ponnusamy S, Sandhiya L, Senthilkumar K (2017a) The atmospheric oxidation mechanism and kinetics of 1, 3, 5-trimethylbenzene initiated by OH radicals–a theoretical study. New J Chem 41:10259–10271
Ponnusamy S, Sandhiya L, Senthilkumar K (2017b) Reaction mechanism and kinetics of the degradation of terbacil initiated by OH radical-a theoretical study. Chem Phys
Rao PK, Gejji SP (2017) Kinetics and mechanistic investigations of atmospheric oxidation of HFO-1345fz by OH radical: insights from theory. J Phys Chem A 121:595–607
Remucal CK (2014) The role of indirect photochemical degradation in the environmental fate of pesticides: a review. Environ Sci: Process Impacts 16:628–653
Ren X, Cui Z, Sun Y (2014) Theoretical studies on degradation mechanism for OH-initiated reactions with diuron in water system. J Mol Model 20:2280
Sandhiya L, Senthilkumar K (2014) Reaction mechanism and kinetics of the degradation of bromoxynil initiated by OH radical. RSC Adv 4:7749–7759
Shiroudi A, Deleuze MS, Canneaux S (2014) Theoretical study of the oxidation mechanisms of naphthalene initiated by hydroxyl radicals: the OH-addition pathway. J Phys Chem A 118:4593–4610
Tanaka F, Hoffer B, Wien R (1986) Photolysis of 3-(3, 4-dichlorophenyl)-1, 1-dimethylurea (diuron) in dilute aqueous solution. Toxicol Environ Chem 11:261–269
Tomlin CD (2009) The pesticide manual: a world compendium. British Crop Production Council
Vereecken L, Francisco JS (2012) Theoretical studies of atmospheric reaction mechanisms in the troposphere. Chem Soc Rev 41:6259–6293
Waite DT, Cessna AJ, Gurprasad NP, Banner J (1999) A new sampler for collecting separate dry and wet atmospheric depositions of trace organic chemicals. Atmos Environ 33:1513–1523
Zhang F, Liu B, Liu G, Zhang Y, Wang J, Wang S (2018) Substructure-activity relationship studies on antibody recognition for phenylurea compounds using competitive immunoassay and computational chemistry. Sci Rep 8:3131
Zhao Y, Truhlar DG (2004) Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: the MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J Phys Chem A 108:6908–6918
Zhao Y, Truhlar DG (2008) Density functionals with broad applicability in chemistry. Acc Chem Res 41:157–167
Zheng J, Bao J, Meana-Pañeda R, Zhang S, Lynch B, Corchado J, Chuang Y, Fast P, Hu W, Liu Y (2016a) Polyrate-version 2016-2A. University of Minnesota, Minneapolis
Zheng J, Zhang S, Corchado J, Chuang Y, Coitiño E, Ellingson B, Truhlar D (2016b) GAUSSRATE version 2016. University of Minnesota, Minneapolis
Funding
The authors received funding from UGC and the Department of Science and Technology (DST), India, to establish high-performance computing facility under the SAP and PURSE programs.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vítor Pais Vilar
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Cartesian coordinates of the transition state structures involved in the studied reactions calculated at M06-2X/6-31G(d,p) level of theory are summarized in Table S1. The calculated relative energy (∆E, kcal/mol), enthalpy (∆H298, kcal/mol) and Gibbs free energy (∆G298, kcal/mol) for the initial OH addition reaction of diuron are given in Table S2. Relative energy (∆E, kcal/mol), enthalpy (∆H, kcal/mol) and Gibbs free energy (∆G, kcal/mol) of the reactive species involved in the initial OH addition reactions of diuron by OH radical in aqueous medium obtained at M06-2X/6-31G(d,p) level of theory are given in Table S3. The rate constant for the initial reactions calculated using CVT, TST and TST (SCT) methods are summarized in Table S4. The relative energy (∆E), enthalpy (∆H298) and Gibbs free energy (∆G298) of the reactive species involved in the reaction of intermediate, I4 with O2 are given in Table S5. The relative energy (∆E), enthalpy (∆H298) and Gibbs free energy (∆G298) of all the reactive species involved in the subsequent reactions of intermediate, I7 are given in Table S6. The relative energy (∆E), enthalpy (∆H298) and Gibbs free energy (∆G298) of all the reactive species involved in the subsequent reactions of intermediate, I8 are given in Table S7. The optimized geometry of reactant complexes, intermediates and products involved in the OH addition reaction of diuron are shown in Fig. S1. The structure of reactant complexes, intermediates and product for the reaction of I4 with O2 are shown in Fig. S2. The optimized structure of intermediates, products involved in the subsequent reactions of intermediate, I12 are shown in Fig. S3. The optimized structure of intermediates and products involved in the subsequent reactions of intermediate, I8 are shown in Fig. S4. (DOCX 934 kb)
Rights and permissions
About this article
Cite this article
Manonmani, G., Sandhiya, L. & Senthilkumar, K. Mechanism and kinetics of diuron oxidation by hydroxyl radical addition reaction. Environ Sci Pollut Res 27, 12080–12095 (2020). https://doi.org/10.1007/s11356-020-07806-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-020-07806-4